The ideal tumor treatment not only destroys the primary tumor, but also improves the immunogenicity of the tumor microenvironment in order to achieve a good anti-tumor immune effect. To this end, Mao Zongwan of Sun Yat-sen University designed a rhenium(I) photosensitizer that anchors carbonic anhydrase IX (CAIX), named CA-Re, which can not only efficiently perform type I and type II light under hypoxia. Dynamic therapy (PDT), and can induce gasdermin D (GSDMD) to mediate pyrolysis, effectively stimulating tumor immunogenicity. CA-Re can simultaneously destroy and self-report the loss of membrane integrity. It promotes the maturation and antigen presentation of dendritic cells (DCs), and fully activates the T cell-dependent adaptive immune response in the body, and ultimately eliminates the primary tumor at the same time as the remote tumor. CA-Re is the first pyrolysis inducer based on metal complexes. This research provides a new perspective for metal immunotherapy of tumors.
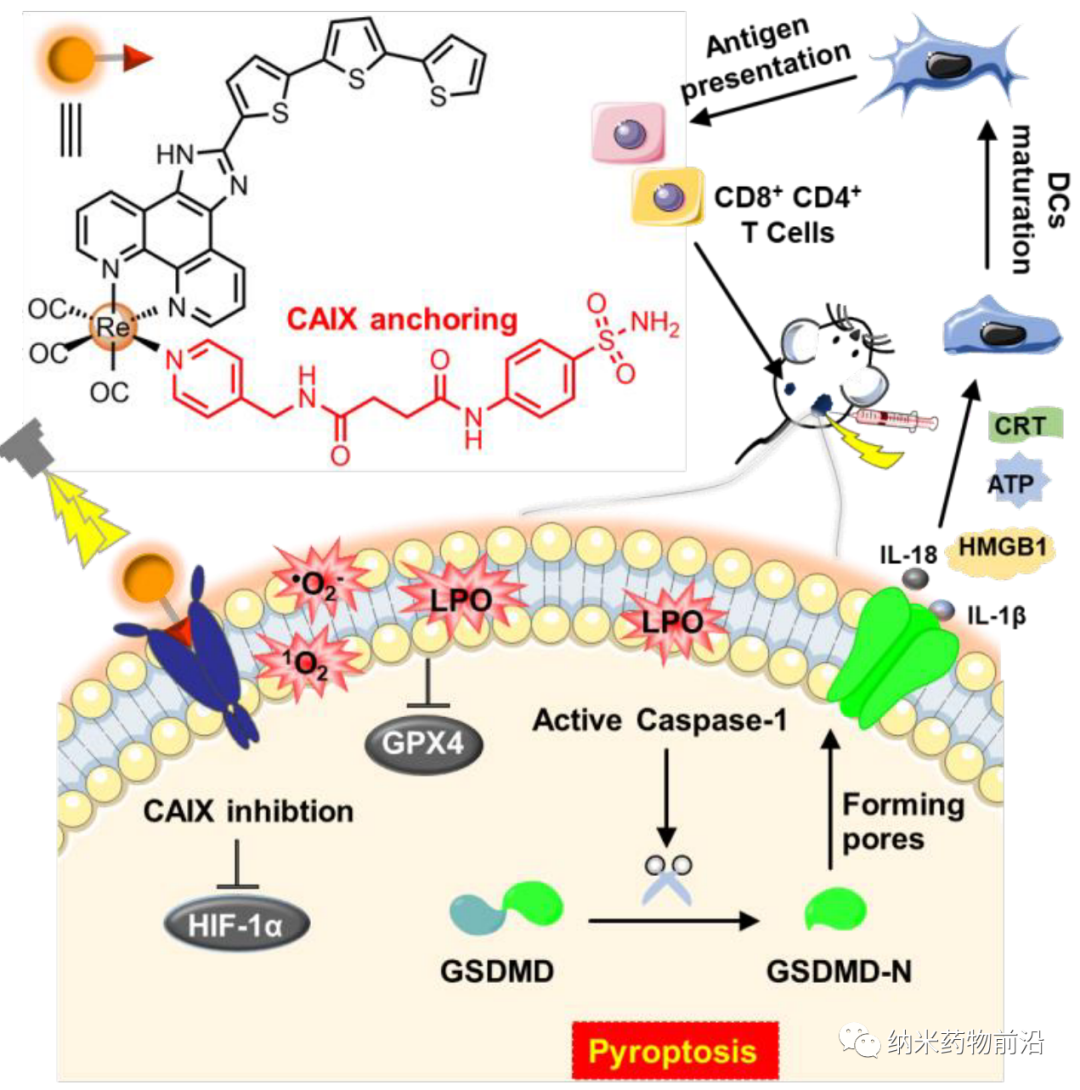
With the clinical success of immune checkpoint blocking therapy (ICB) in some advanced cancers, immunotherapy has attracted widespread attention. However, its therapeutic effect on malignant tumors such as triple negative breast cancer (mTNBC) and colorectal cancer is severely limited by the non-immunogenic tumor microenvironment. In recent years, there has been a lot of evidence that photodynamic therapy (PDT) will produce immune stimulation, suggesting that it is a potential immunogenic treatment. PDT is a kind of use of light and non-toxic photosensitizer to produce singlet oxygen (1O2) through the oxygen-dependent pathway (type-II), or through the oxygen-independent pathway (type-I) to produce other reactive oxygen species (ROS), with time and A non-invasive clinical treatment method for spatially selective destruction of tumor tissue. In some cases, PDT can induce cells to release damage-related molecules (DAMPs), thereby enhancing the immunogenicity of cancer cells and activating the systemic adaptive immune response. However, most photosensitizers must be used in combination with ICB (such as anti-CTAL4, anti-PD-L1) to achieve better anti-tumor immunity, but the cost is high and the side effects are serious. The main reasons for its lack of universality and effectiveness are: (1) Tumor hypoxia severely limits the efficiency of PDT and promotes immunosuppression; (2) PDT usually induces cell apoptosis. Due to the slow or even no release of DAMPs, its immunity Poor originality. Therefore, in order to achieve effective anti-tumor immunity, there is an urgent need for new photosensitizers that can achieve high PDT efficiency under hypoxic conditions and induce cell death instead of apoptosis with high immunogenicity. Pyrolysis is a unique form of programmed cell death. Inflammatory caspases cleavage gasdermin D (GSDMD) is considered to be a key event that mediates pyrolysis, which releases the N-terminal domain (GSDMD-N) to bind to membrane phospholipids and drill holes in the cell membrane. As a result, the osmotic potential of the cell membrane is destroyed, leading to cell swelling, cell membrane rupture, and rapid release of inflammatory factors (such as IL-1β, IL-18). Studies have found that the inflammatory response caused by pyrolysis can stimulate strong anti-tumor immunity and enhance the efficacy of ICB. Therefore, scorch provides a new direction for the design of highly immunogenic photosensitizers.
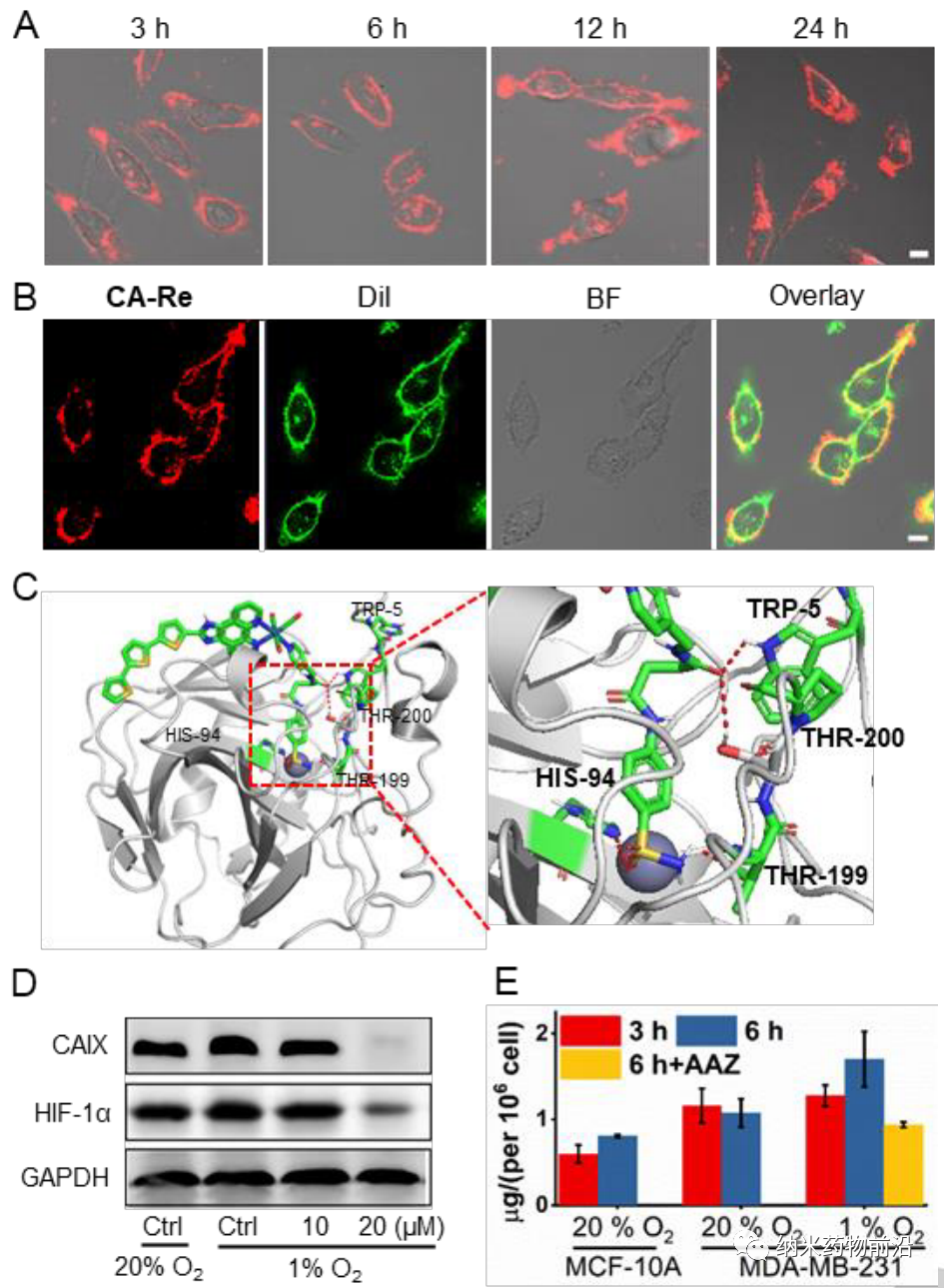
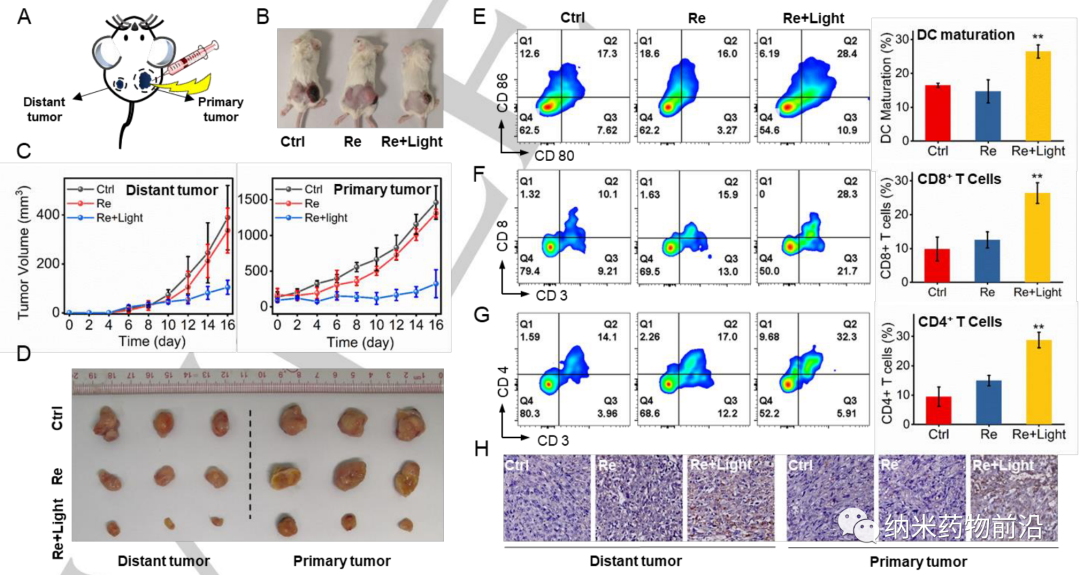
Because CAIX is a transmembrane protein, it is only overexpressed in tumors and not in normal tissues. Its transcription is regulated by hypoxia-inducible factor (HIF-1α). The author designed a pyridyl ligand with a benzenesulfonamide tail, which is a common part of CAIX to bind to the zinc active center. Then the monodentate ligand was reacted with the dechlorinated Re(CO)3(N^N)Cl precursor in tetrahydrofuran to synthesize the new photosensitizer CA-Re, and then purified by column chromatography (CH2Cl2/CH3OH). The characterization and properties results show that CA-Re has excellent photostability and does not release pyridine ligand even after irradiation. In addition, CA-Re possesses CAIX anchoring and intervention capabilities, enabling it to be fixed on the cell membrane and relieve tumor hypoxia to a certain extent. This promotes the production of in situ ROS and lipid peroxidation on the membrane, and finally exhibits extremely high PDT efficiency (phototoxicity at the nanomolar level) under hypoxic conditions, and effectively triggers gasdermin D-mediated pyrolysis . During this process, CA-Re caused and self-reported the loss of cell membrane integrity. Therefore, a series of inflammatory factors and DAMPs are rapidly released at the treatment site, which stimulates the maturation of dendritic cells and the presentation of antigens, and finally activates the T cell-dependent adaptive immune response in the body, destroying the primary tumor while inhibiting the development of distant tumors. Grow.
Original link:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202115800
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.






