Chen Qian/Liu Zhuang Nano Lett: Anti-inflammatory nanofiber hydrogel for injection can achieve systemic immunotherapy after topical administration


Although immune checkpoint blockade (ICB) therapy has made great promise in using the immune system to combat different tumors, limitations such as low objective response rates and adverse reactions still need to be resolved. Here, Professor Liu Zhuang and Professor Chen Qian from the Institute of Functional Nano and Soft Matter of Soochow University have developed an anti-inflammatory nanofiber hydrogel self-assembled through steroid drugs for the local delivery of anti-apoptotic protein ligand 1 (αPDL1). ).
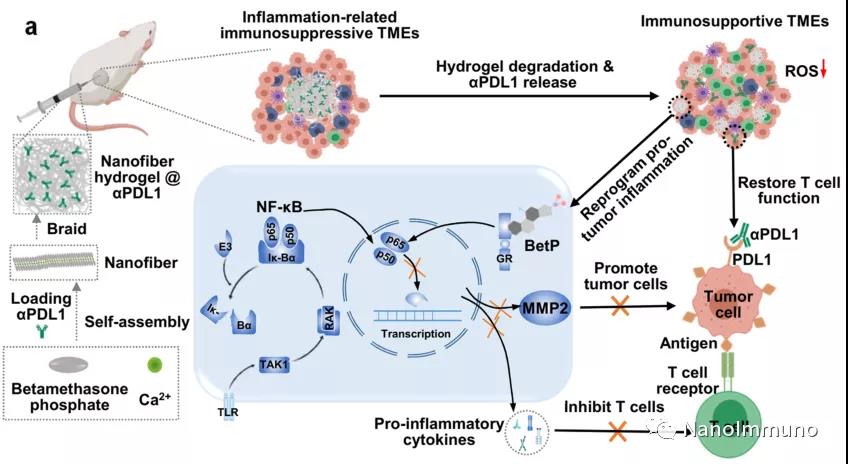

On the one hand, this carrier-free system based on steroid drugs can reprogram the immunosuppressive tumor microenvironment (TME) into an anti-tumor TME; on the other hand, it will act as a reservoir for the sustained release of αPDL1 and synergistically enhance the immune system. By locally injecting this hydrogel loaded with αPDL1, without any treatment, effective therapeutic effects have been observed in inhibiting local tumors and distant tumors. This work proposes a unique hydrogel-based drug delivery system that uses clinically approved drugs, showing an improved objective response rate of ICB treatment and minimizing its systemic toxicity.


The injectable shear-thinning anti-inflammatory hydrogel is achieved by combining BetP (a clinically approved steroid anti-inflammatory drug) with calcium ions through non-covalent interactions (including intermolecular hydrogen bonds, hydrophobic interactions, and phosphate groups). The coordination) is mixed and self-assembled. This nanofiber hydrogel can be used as a carrier-free delivery platform for effective delivery of ICB antibodies (such as αPDL1) after local injection, thereby achieving long-term retention of encapsulated antibodies. Through the competitive coordination interaction between BetP and phosphate under physiological conditions, the nanofiber hydrogel locally injected into mice can be gradually degraded to release BetP and encapsulated antibodies.
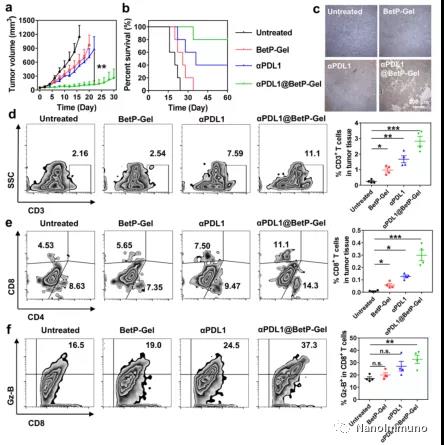
This drug-based topical nanofiber hydrogel can enhance the anti-tumor immune response. The hydrogel itself can reprogram inflammation-related immunosuppressive TME by clearing chronic inflammation, including attenuating inflammation-related NF-κB signaling pathways and reducing the expression levels of pro-inflammatory cytokines and MMP2. The reprogrammed inflammation-related TME is characterized by improved tumor infiltration of T lymphocytes and a decrease in the percentage of immunosuppressive cells. On the other hand, this nanofiber hydrogel can be used as an effective reservoir, allowing the local and sustained release of αPDL1, thereby avoiding the systemic toxicity of ICB. In addition, the sustained release of αPDL1 can effectively reverse T cell exhaustion for a long time, activate T cell-mediated immune attack of cancer cells, and inhibit the growth of primary and distant tumors.


This article has proved that local injection of αPDL1@BetP-Gel in CT26 tumor model can effectively inhibit the growth of primary and distal tumors. Current technologies and formulations have great potential in clinical transformation. Such immunotherapeutic nanofiber hydrogels are made from a simple mixture of clinically used steroid drugs, antibodies and calcium ions. The hydrogel with shear thinning capability allows it to be injected directly into the tissue with minimal invasiveness. Therefore, considering its composition with all clinically approved ingredients, a fairly simple and easy-to-scale preparation process, minimally invasive drug delivery methods, and local controlled release delivery of αPDL1 with low systemic toxicity but systemic therapeutic effects, this strategy has It has great potential in the treatment of advanced metastatic cancer in the clinic.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
18915694570
Previous: A review by Wang Xiany


