Non-enzymatic glucose sensor based on porous foam gold/MXene nanocomposite
I. Article overview
An electrochemical non-enzymatic glucose sensor based on three-dimensional Au/MXene nanocomposites was developed. MXene was prepared by a gentle etching method, and the porous foam of gold nanoparticles was combined with MXene by in-situ synthesis. During the synthesis process, by controlling the quality of MXene, a porous foam material containing gold nanoparticles was obtained. Scanning electron microscopy confirmed the three-dimensional foam structure of the nanoparticles. Cyclic voltammetry and electrochemical impedance spectroscopy were used to study the electrochemical properties of gold/MXene nanocomposites. Au/MXene nanocomposite has good performance as a rapid redox probe for non-enzymatic glucose oxidation. Its sensitivity is 22.45μA·(mmol/L)-1·cmm -1, and the linear range is 1-12 mmol/L. . Studies have shown that MXene as a catalyst supporting material is beneficial to improve the conductivity of electrons and increase the loading rate of the catalyst material. The foam structure containing gold nanoparticles can provide a larger surface area, increase the contact area with molecules in the catalytic reaction, and enhance the electrochemical reaction signal. In summary, the research in this article shows that gold/MXene nanoparticles have the potential to be used in non-enzymatic glucose sensors.
II.Graphic guide
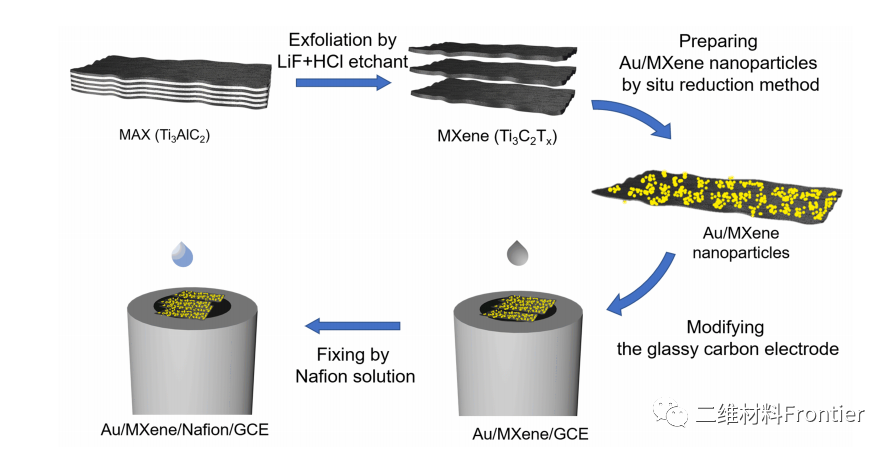
Figure 1. Preparation process of gold/MXene/Nafion/GCE electrode.
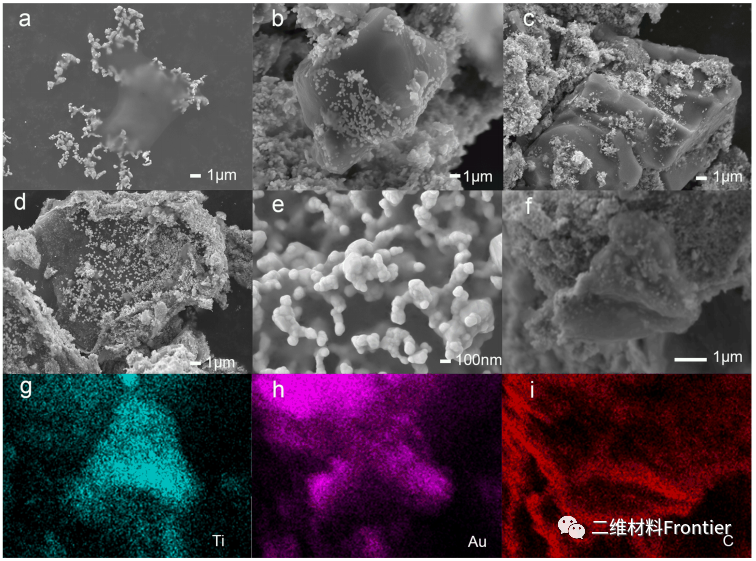
Figure 2. (a) Scanning electron microscope (SEM) images of Au/MXene composite nanoparticles prepared from 1μLMXene suspension, (b)2.5μLMXene suspension, (c)5μLMXene suspension, and (d)10μLMXene suspension; (e) ) Scanning electron microscope image of porous foam structure on the surface of multilayer MXene nanoparticles; (f) Scanning electron microscope image of Au/MXene nanocomposite material. Elemental mapping of gold/MXene composite nanoparticles: (g) Ti, (h) Au, (i) C.
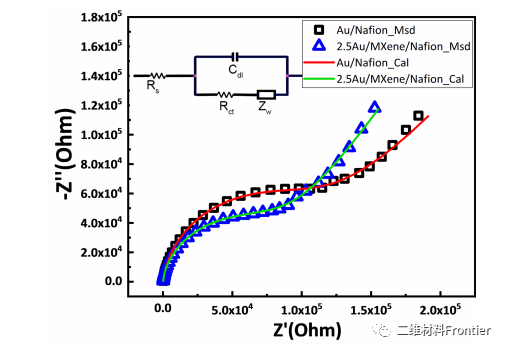
Figure 3. Electrochemical impedance spectroscopy (EIS) of Au/Nafion electrode and Au/MXene/Nafion electrode and its fitting result The illustration shows the Randle equivalent circuit of the EIS test.
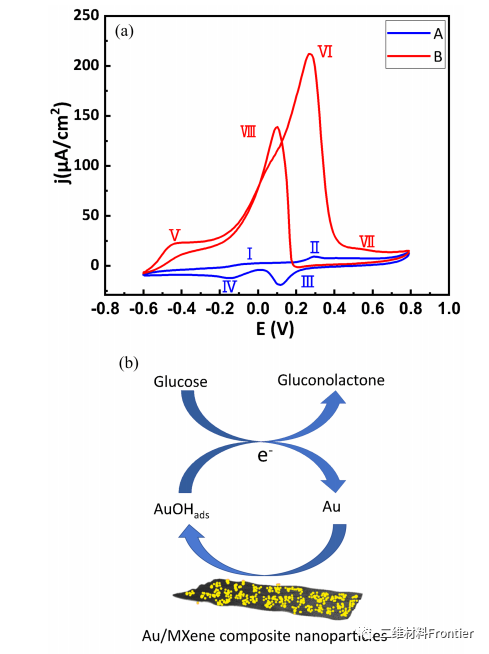
Figure 4. (a) CV scan of Au/Nafien/GCE at 40 mV/s in 0.1 mol/L sodium hydroxide solution (curve A: without 10 mmol/L glucose, curve B: with 10 mmol/L glucose). (b) Schematic diagram of the electrocatalytic process of Au/MXene/Nafion/GCE oxidation of glucose.
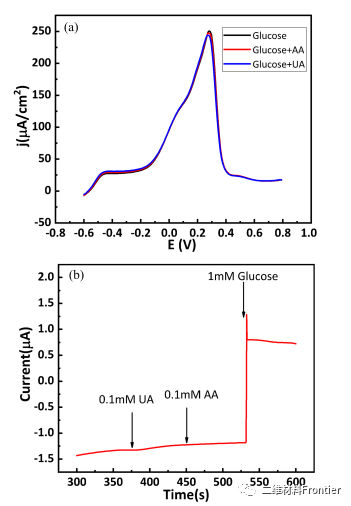
Figure 5. (a) Scanning of interfering substances (0.1mmol/L ascorbic acid (AA) and 0.1mmol/L uric acid (UA) in 10mmol/L glucose, Au/MXene/Nafion/GCE in 0.1mol/L sodium hydroxide solution The scan rate is 40mV/s; (b) Ampere reaction of Au/MXene/Nafion/GCE in the presence of 0.1mmol/LAA and 0.1mmol/LUA. The detection potential is 0.1V.
III. Full text summary
This work demonstrated the application of Au/MXene in non-enzymatic glucose sensors and evaluated the performance of MXene as a substrate and porous gold nanoparticles as a glucose catalyst. A simple chemical reduction method was used to prepare three-dimensional gold/MXene nanocomposites, and the morphology of porous gold nanoparticles was controlled by the quality of MXene. The Au/MXene/Nafion/GCE biosensor has a good detection effect on glucose. The linear range is 1 -12 mmol/L (R2=0.998), and the peak current is 22.45μA·(mmol/L)- 1·cmm -1. The limit is 0.2 mmol/L (S/N=3). In addition, the Au/MXene/Nafion/GCE sensor is also selective for a variety of interfering substances. It can be stored at 4°C for 7 days, and the sensitivity remains 88%. The most obvious finding of this article is that MXene as a substrate improves the electrochemical activity of non-enzymatic glucose sensors in alkaline solutions. In summary, these results indicate that Au/MXene composite nanoparticles are a potential material in constructing non-enzymatic glucose biosensors.
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
18915694570
Previous: Lu Ligong/Gao Huile AF


