
Protein therapy has great potential in disease treatment due to its pharmacological efficacy and specificity. However, because hydrophilic protein drugs with high molecular weight are impermeable to cell membranes, the clinical availability of protein therapy is limited to extracellular targets. Although it is now proposed that proteins with intracellular targeting have better therapeutic results, the success of intracellular protein therapy is greatly limited due to the lack of effective cytosolic protein delivery strategies.
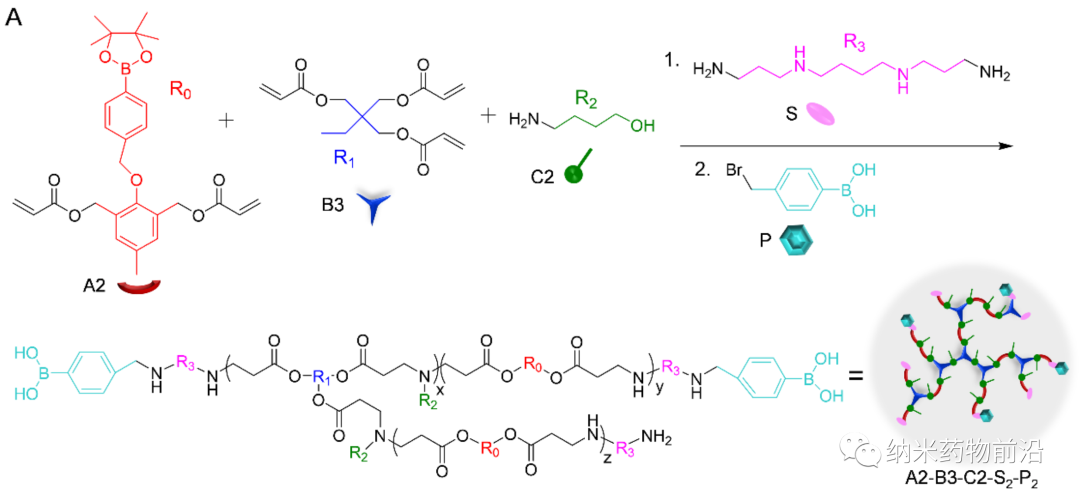
Cytosolic protein delivery is a prerequisite for protein-based biotechnology and intracellular target therapy. The key process is to effectively encapsulate the protein drug into the cell-ingestible nanosystem. A more mature strategy is to use nanocarriers, such as liposomes, polymer vesicles, nanocapsules, nanoemulsions, mesoporous silica nanoparticles (MSNs), etc., to load protein drugs into internal water cavities or pores. However, these methods usually have limitations, such as complex synthesis, low protein loading efficiency, need for purification, destruction of protein structure and biological activity, and so on. Another method is to covalently modify the protein and anionic substances, such as carboxylic acid, boric acid or other anionic proteins, so that the modified protein can form nanocomposites (NC) with cationic materials through electrostatic interactions. Although these chemically modified parts can be removed by stimulating factors in the cell, they often affect the biological activity of the protein. In addition, polymer-mediated cytoplasmic delivery of proteins has also emerged. Use hydrogen bond between polymer and protein, hydrophobic interaction, metal coordination interaction, nitrogen borate (N-B) coordination interaction and other self-assembly to form a stable NC. Therefore, various polymers, such as guanidine-rich polymers, boric acid-rich polymers, etc., have been designed for cytoplasmic protein delivery. However, due to the strong binding force, it will inevitably delay the release of intracellular proteins and therefore reduce protein activity.
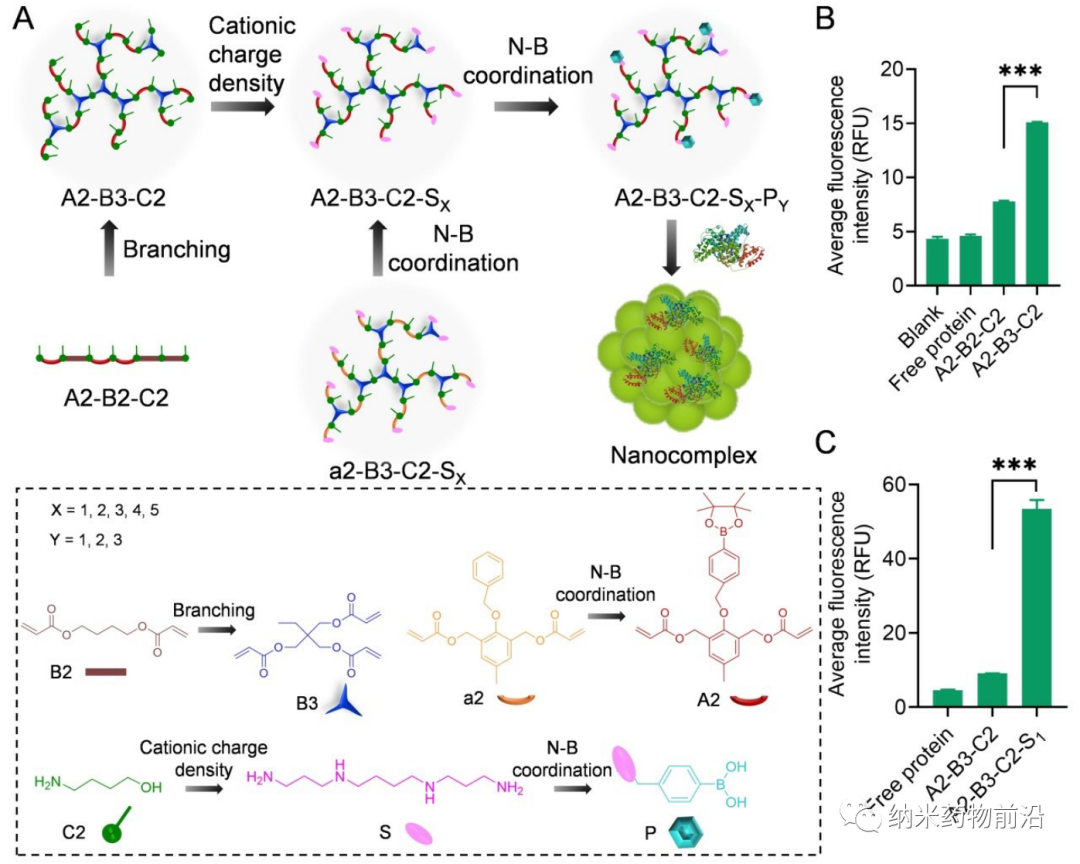
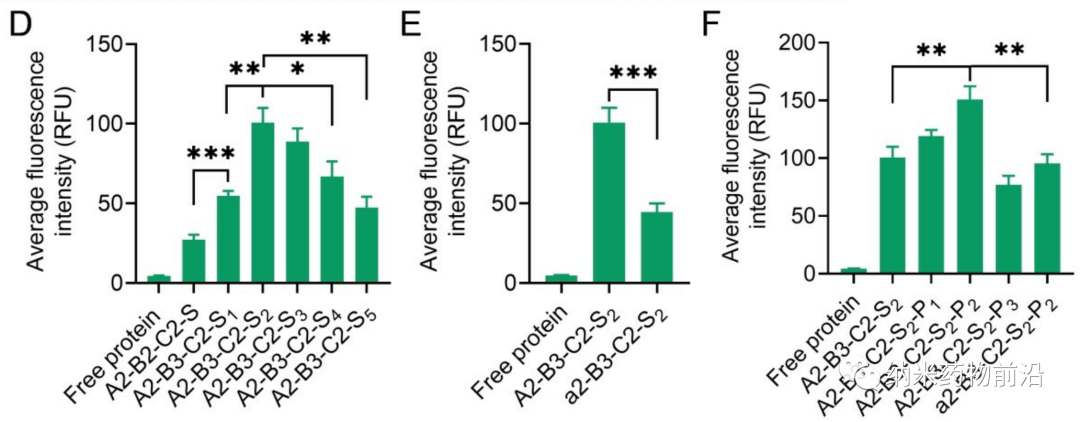
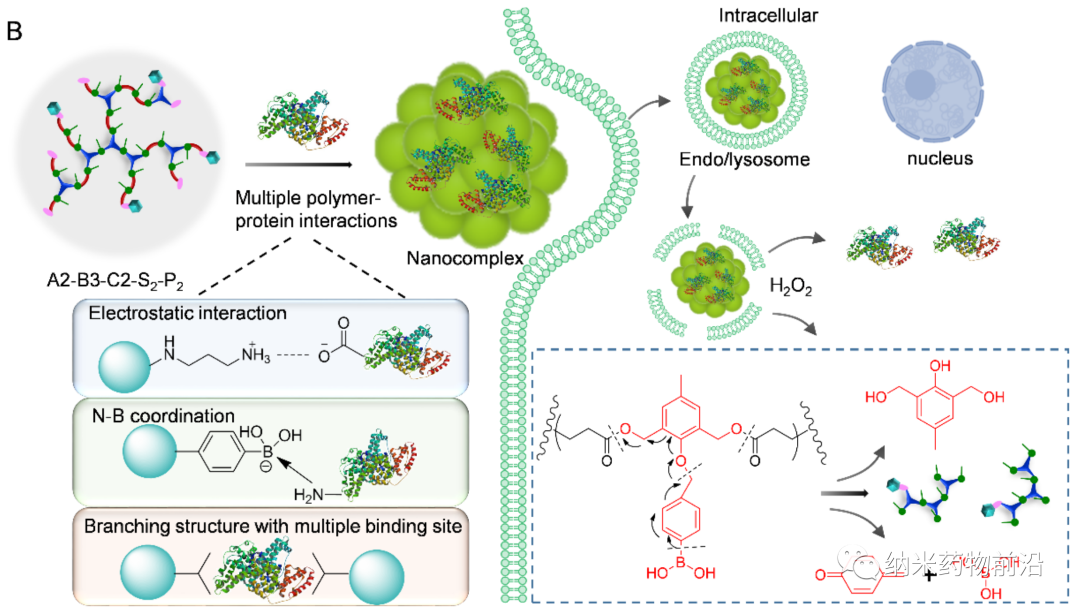
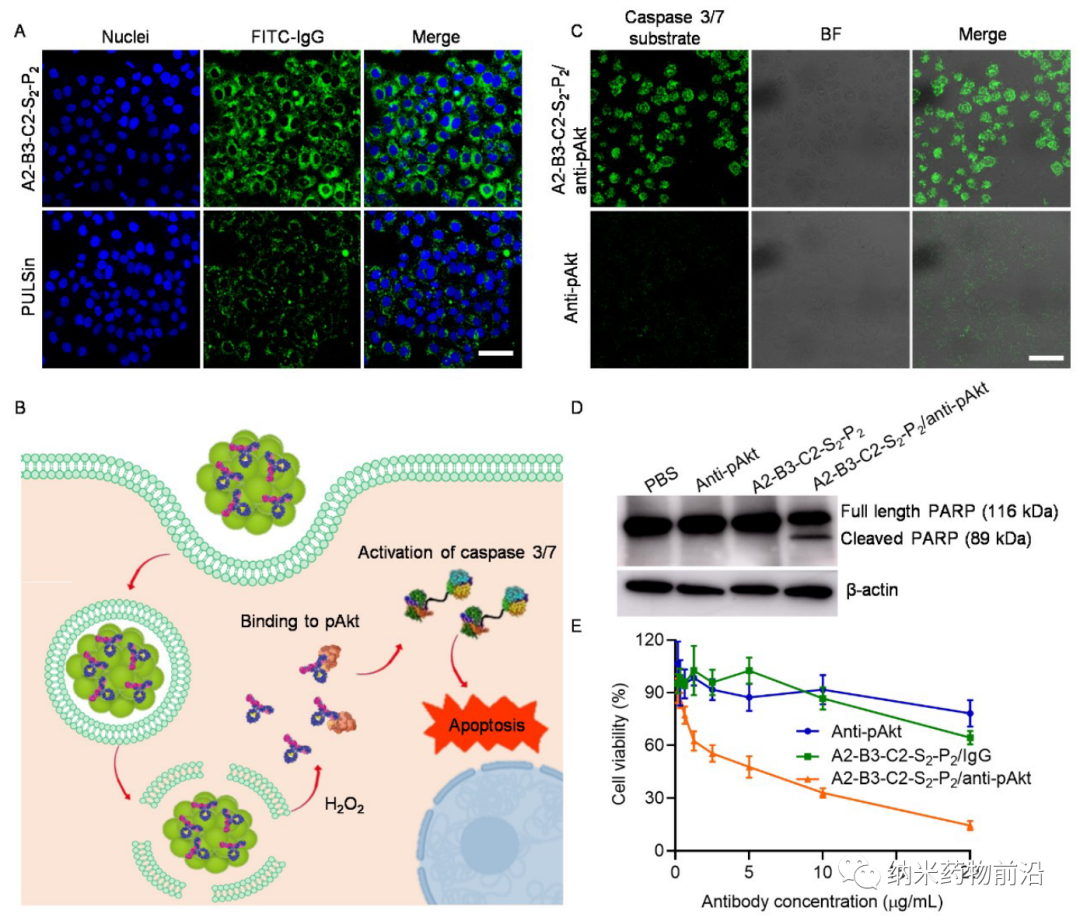
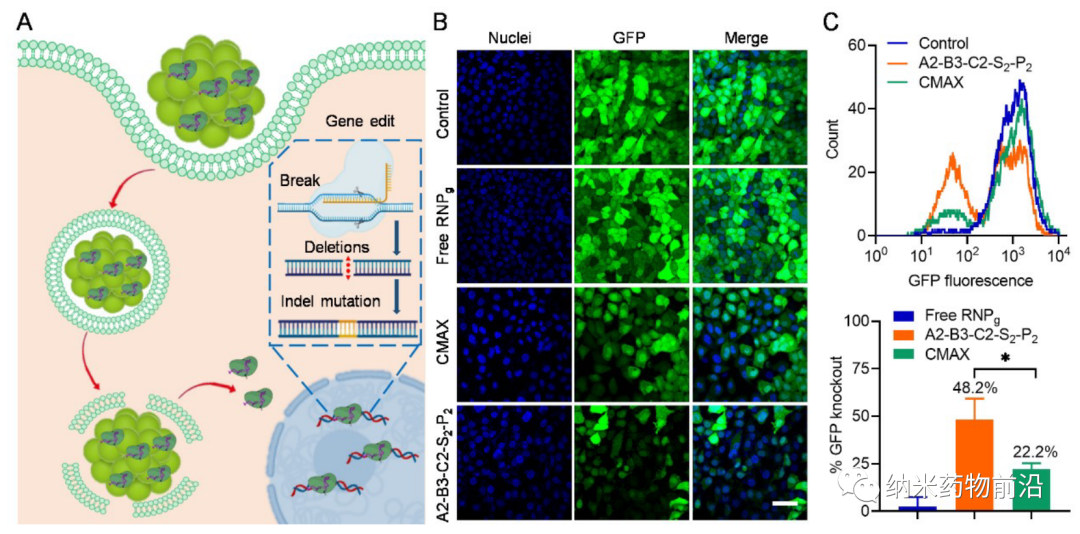
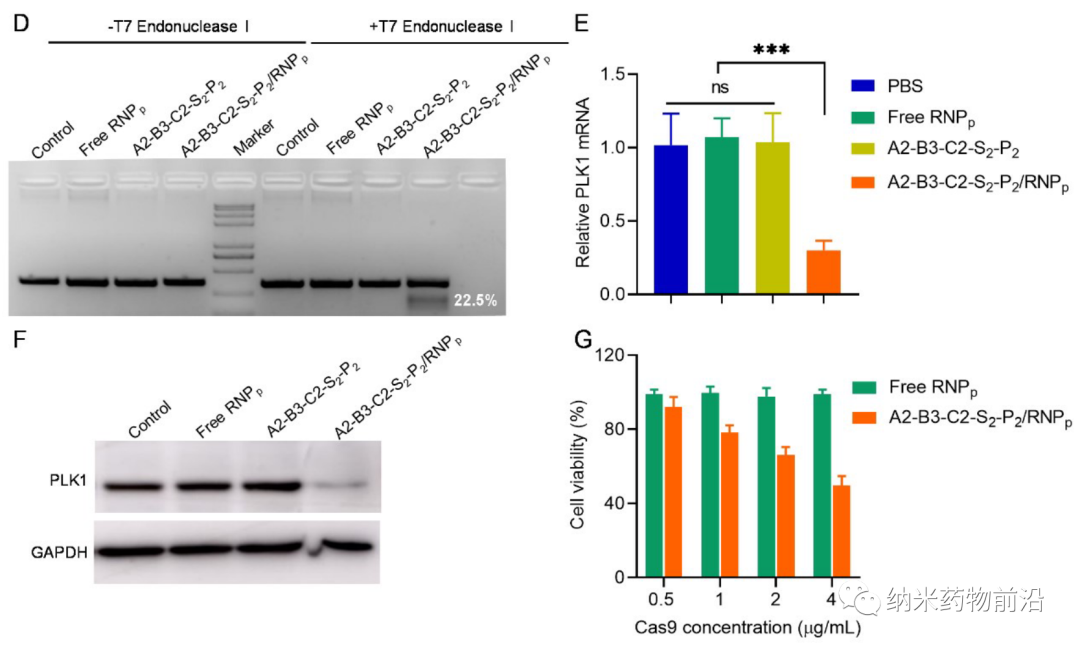
In order to solve the problems caused by the above methods, Professor Yin Lichen of Soochow University designed a universal cytoplasmic protein delivery platform based on ROS-degradable hyperbranched poly(β-aminoester) (HPAE) backbone grafted with phenylboronic acid (PBA). The branched structure makes possible multivalent enhanced protein binding, and uses electrostatic interactions to further enhance protein binding through spermine end-capping. The PBA in the backbone binds to the protein drug through N-B coordination, and the additional use of PBA end-capping further enhances this binding force. Because cancer cells can produce more reactive oxygen species (ROS), such as peroxides, superoxides, and hydroxyl radicals. Therefore, in the cytoplasm of cancer cells, the skeleton-grafted PBA is oxidized by excess H2O2, which triggers rapid degradation of the polymer and release of biologically active proteins. Experiments show that this strategy can mediate the powerful delivery of a large number of proteins or polypeptides with different molecular weights and isoelectric points to cancer cells, including enzymes, antibodies, CRISPR-Cas9 ribonucleoprotein, etc., and can effectively deliver saponin in the body. Causes significant anti-tumor efficacy.
Original link:
https://onlinelibrary.wiley.com/doi/10.1002/adma.202108116
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.










