KAUST AFM: Covalent assembly of MXene@COF two-dimensional nanosheets and its application in lithium batteries

Article information
Covalently assembled two-dimensional MXene@COF heterojunction to achieve stable lithium metal anode First author: Dong Guo (郭冬) Corresponding author: Zhiping Lai (赖志平) Professor: King Abdullah University of Science and Technology, Saudi Arabia
Research Background
Lithium metal has high specific capacity and low potential and is regarded as the most promising negative electrode material in lithium batteries. However, the growth of lithium dendrites seriously affects the stability of the lithium metal negative electrode and the safety of the battery. This paper presents a class of three-dimensional lithium metal host materials composed of two-dimensional heterostructures, which show unique advantages in achieving dendritic-free lithium metal deposition.
Article Introduction
Based on this, the team of Professor Lai Zhiping from King University of Science and Technology (KAUST) in Saudi Arabia published an article entitled "Covalent Assembly of Two-Dimensional COF-on-MXene Heterostructures Enables Fast Charging Lithium Hosts" in the internationally renowned journal Advanced Functional Materials. . The article pointed out that by covalently growing a two-dimensional covalent organic framework (COF) on a conductive ultra-thin two-dimensional MXene nanosheet, a new type of two-dimensional organic/inorganic heterogeneous nanosheet structure was realized. Experiments have shown that such a large number of nano-scale two-dimensional COF upper pores play an important role in regulating the uniform transfer of lithium ions across the electrode. At the same time, the high electronic conductivity of MXene improves the deposition kinetics of lithium ions on the entire electrode and avoids charge accumulation. In addition, theoretical calculation combined with kinetic analysis showed that the C=N group in the COF channel has undergone a certain degree of lithiation during the discharge process. This lithiation before lithium deposition makes the entire porous nanosheet have good lithium affinity, thereby achieving a very low lithium nucleation overpotential.
Highlights of this article
Key point 1: Synthesis of MXene@COF nanosheets with porous two-dimensional interface based on MXene. This article reports for the first time the possibility of covalently growing two-dimensional COF on two-dimensional conductive MXene. First, the hydrolysis reaction is used to aminate the surface of MXene. Subsequently, by regulating the Schiff base reaction, the uniform growth of COF-LZU1 on MXene was achieved.
Covalently assembled two-dimensional MXene@COF heterojunction to achieve stable lithium metal anode First author: Dong Guo (郭冬) Corresponding author: Zhiping Lai (赖志平) Professor: King Abdullah University of Science and Technology, Saudi Arabia
Research Background
Lithium metal has high specific capacity and low potential and is regarded as the most promising negative electrode material in lithium batteries. However, the growth of lithium dendrites seriously affects the stability of the lithium metal negative electrode and the safety of the battery. This paper presents a class of three-dimensional lithium metal host materials composed of two-dimensional heterostructures, which show unique advantages in achieving dendritic-free lithium metal deposition.
Article Introduction
Based on this, the team of Professor Lai Zhiping from King University of Science and Technology (KAUST) in Saudi Arabia published an article entitled "Covalent Assembly of Two-Dimensional COF-on-MXene Heterostructures Enables Fast Charging Lithium Hosts" in the internationally renowned journal Advanced Functional Materials. . The article pointed out that by covalently growing a two-dimensional covalent organic framework (COF) on a conductive ultra-thin two-dimensional MXene nanosheet, a new type of two-dimensional organic/inorganic heterogeneous nanosheet structure was realized. Experiments have shown that such a large number of nano-scale two-dimensional COF upper pores play an important role in regulating the uniform transfer of lithium ions across the electrode. At the same time, the high electronic conductivity of MXene improves the deposition kinetics of lithium ions on the entire electrode and avoids charge accumulation. In addition, theoretical calculation combined with kinetic analysis showed that the C=N group in the COF channel has undergone a certain degree of lithiation during the discharge process. This lithiation before lithium deposition makes the entire porous nanosheet have good lithium affinity, thereby achieving a very low lithium nucleation overpotential.
Highlights of this article
Key point 1: Synthesis of MXene@COF nanosheets with porous two-dimensional interface based on MXene. This article reports for the first time the possibility of covalently growing two-dimensional COF on two-dimensional conductive MXene. First, the hydrolysis reaction is used to aminate the surface of MXene. Subsequently, by regulating the Schiff base reaction, the uniform growth of COF-LZU1 on MXene was achieved.
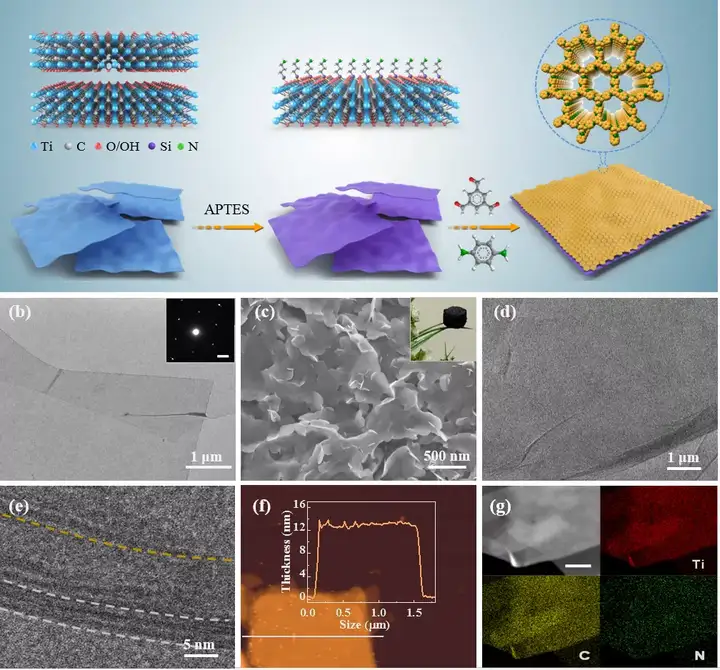
Figure 1. Synthetic schematic diagram of MXene@COF heterostructure and morphological characterization of related nanosheets.
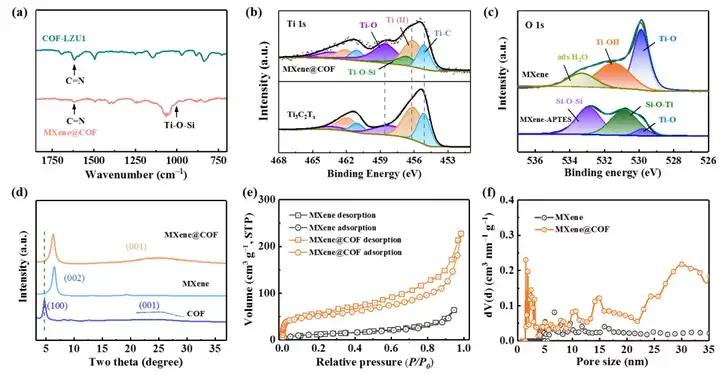
Figure 2. (a-d) Structural and physical characterization of MXene@COF heterostructure. (e-f) Characterization of the pore structure of the three-dimensional Li host formed by the MXene@COF heterostructure.
Point 2: Explored the kinetic mechanism of lithium ion transport and deposition in MXene@COF. The COF channel introduced to achieve a uniform distribution of lithium ions on the electrode at the molecular level has a decisive effect on the dendrite-free lithium deposition. At the same time, theoretical calculations and electrode characterization analysis after cycling show that the COF channels grown on the surface of MXene have a certain lithiation effect during the lithium deposition process. This process achieves a lower nucleation overpotential, thereby avoiding the charge accumulation of lithium ions at high current densities, thereby achieving fast charging without lithium dendrites growth.
Point 2: Explored the kinetic mechanism of lithium ion transport and deposition in MXene@COF. The COF channel introduced to achieve a uniform distribution of lithium ions on the electrode at the molecular level has a decisive effect on the dendrite-free lithium deposition. At the same time, theoretical calculations and electrode characterization analysis after cycling show that the COF channels grown on the surface of MXene have a certain lithiation effect during the lithium deposition process. This process achieves a lower nucleation overpotential, thereby avoiding the charge accumulation of lithium ions at high current densities, thereby achieving fast charging without lithium dendrites growth.
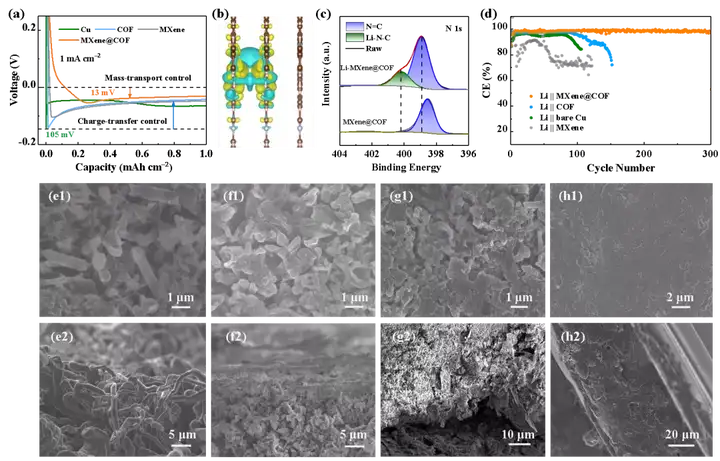
Figure 3. (a-d) Deposition kinetic behavior analysis of lithium ions on 3D MXene@COF. (E-h) Comparison of the deposition morphology of lithium ions in different host materials.
Electrochemical analysis shows that the deposition of lithium ions on MXene@COF nanosheets has very small nucleation overpotential and deposition overpotential. These two smaller potentials confirm the advantage of lithium ion transport kinetics within this framework, and provide important support for dendritic-free lithium deposition.
Electrochemical analysis shows that the deposition of lithium ions on MXene@COF nanosheets has very small nucleation overpotential and deposition overpotential. These two smaller potentials confirm the advantage of lithium ion transport kinetics within this framework, and provide important support for dendritic-free lithium deposition.
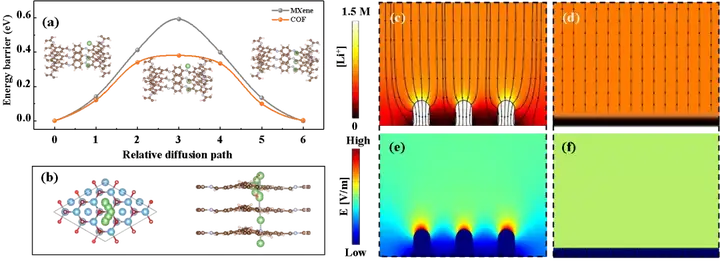
Figure 4. (a-b) Calculation and analysis of the transfer path of lithium ions on MXene and COF nanosheets. (C-f) Lithium ion deposition phase field simulation on Cu (c-d) and MXene@COF.
Key point 3: Constructed excellent Li-S and Li-LFP lithium metal full batteries based on Li/MXene@COF. Using Li-plated Li/MXene@COF as the negative electrode and sulfur and lithium iron phosphate as the positive electrode respectively, the assembly of the full battery is realized. Both types of full batteries exhibit good cycle stability. For example, Li/MXene@COF||S battery can be cycled stably for 150 cycles under the condition of limited lithium content, and the specific capacity is maintained at 750 mAh/g. The construction of a stable lithium negative electrode on the surface is important to the performance of the full battery. significance.
Key point 3: Constructed excellent Li-S and Li-LFP lithium metal full batteries based on Li/MXene@COF. Using Li-plated Li/MXene@COF as the negative electrode and sulfur and lithium iron phosphate as the positive electrode respectively, the assembly of the full battery is realized. Both types of full batteries exhibit good cycle stability. For example, Li/MXene@COF||S battery can be cycled stably for 150 cycles under the condition of limited lithium content, and the specific capacity is maintained at 750 mAh/g. The construction of a stable lithium negative electrode on the surface is important to the performance of the full battery. significance.
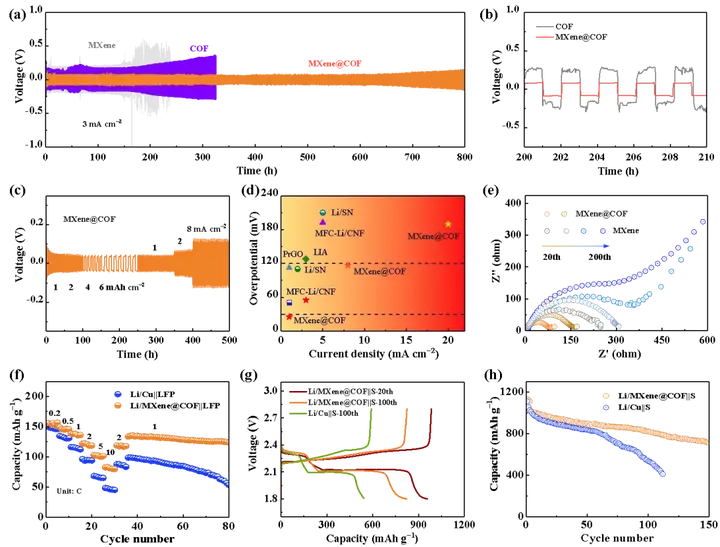
Figure 5. (a-) Cyclic performance characterization of Li/MXene@COF symmetric battery. (F-h) Cycle performance of Li-S and Li-LFP full batteries assembled with Li/MXene@COF as the negative electrode
Article link
Covalent Assembly of Two-Dimensional COF-on-MXene Heterostructures Enables Fast Charging Lithium Hostshttps://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202101194
Corresponding author introduction
Professor Lai Zhiping. Working at King Abdullah University of Science and Technology in Saudi Arabia since 2009. The research work involves the application of porous materials and membrane processes in separation, electrochemical energy conversion and other directions. In recent years, he has published many high-level SCI papers, including Nature Nanotechnology, JACS, Advanced Materials, Energy&Environmental Science, Advanced Functional Materials, Nature Communications, Nano Energy, ACS Nano, etc. Now it has a research team of more than 20 people composed of research scientist, postdoc and PhD student.
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
Article link
Covalent Assembly of Two-Dimensional COF-on-MXene Heterostructures Enables Fast Charging Lithium Hostshttps://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202101194
Corresponding author introduction
Professor Lai Zhiping. Working at King Abdullah University of Science and Technology in Saudi Arabia since 2009. The research work involves the application of porous materials and membrane processes in separation, electrochemical energy conversion and other directions. In recent years, he has published many high-level SCI papers, including Nature Nanotechnology, JACS, Advanced Materials, Energy&Environmental Science, Advanced Functional Materials, Nature Communications, Nano Energy, ACS Nano, etc. Now it has a research team of more than 20 people composed of research scientist, postdoc and PhD student.
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
18915694570
Previous: Artificial Nano Red Bl


