Chen Xiaoyuan/Yang Zhen/Fan Wenpei Nat Biomed Eng: The burst release of Annexin A5 in tumors enhances cytotoxic T cell response by blocking the phagocytosis of apoptotic cells

Cancer immunotherapy, especially therapeutic vaccination, usually does not produce a strong anti-tumor immune response. Here, Chen Xiaoyuan and Yang Zhen from the National Institutes of Health (names in the title are incorrect, I hereby correct them and apologize) and Fan Wenpei of China Pharmaceutical University
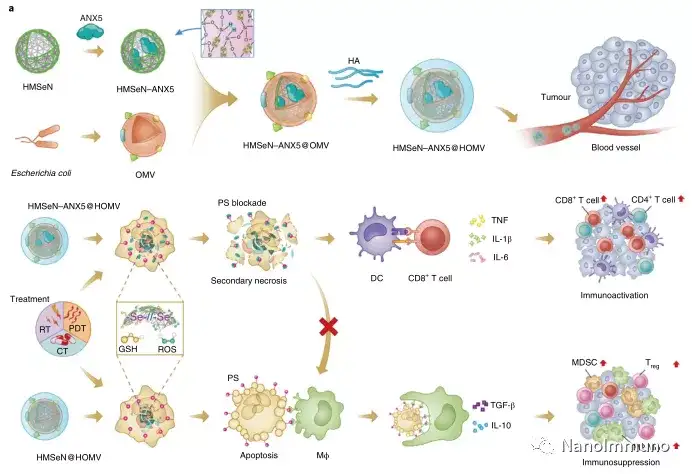

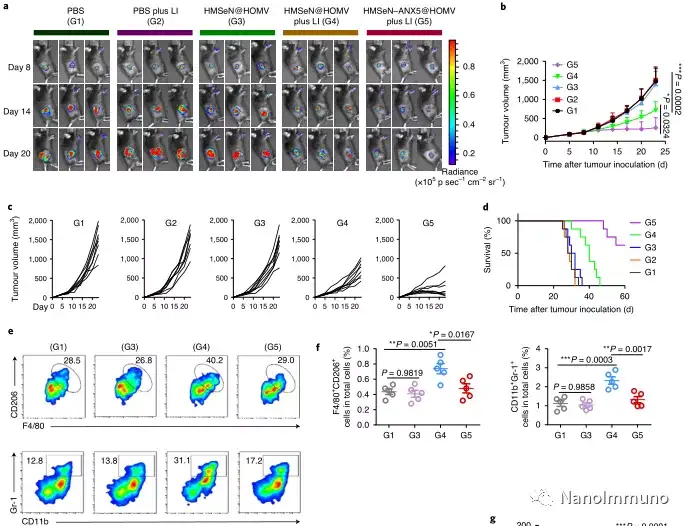
In the tumor mouse model, due to the rupture of the diselenide bond under the oxidative conditions of the tumor microenvironment and the intracellular reduction conditions of the tumor cells, the burst release of Annexin A5 induces systemic cytotoxic T cell responses and interacts with the tumor. The associated immune memory faded away, preventing tumor recurrence and leading to complete eradication of tumors in about 50% of mice with in situ breast tumors. Reducing apoptosis signal transduction through in situ vaccination may be a general strategy for adaptive anti-tumor immune response.
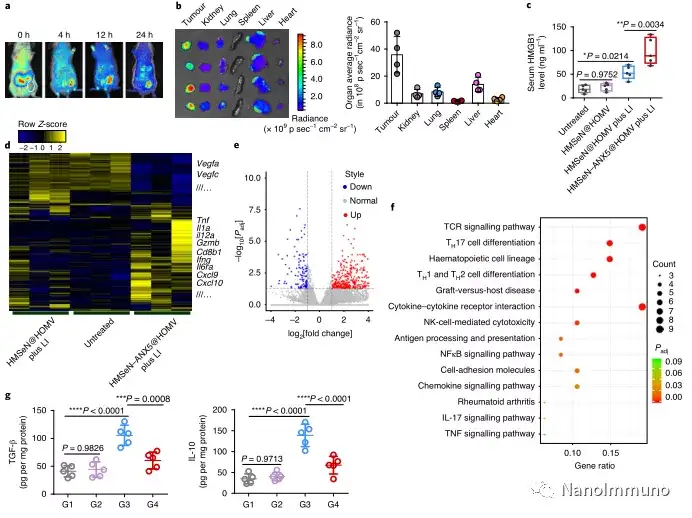
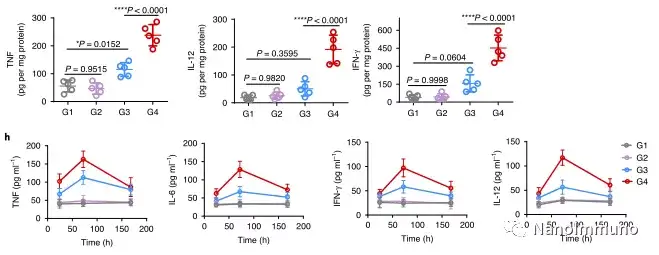
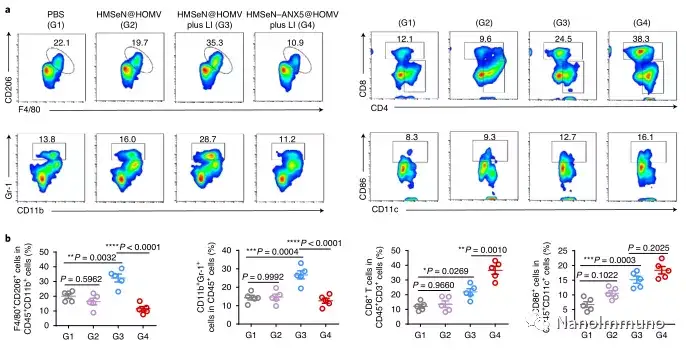
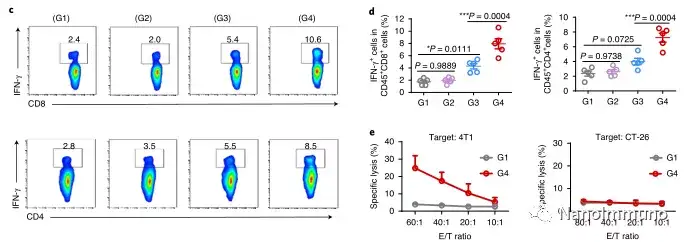
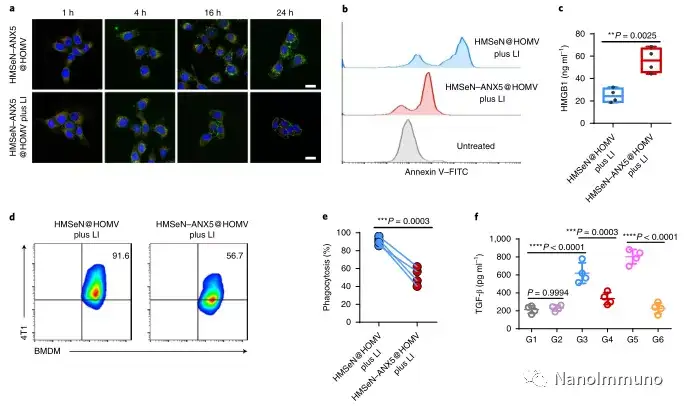
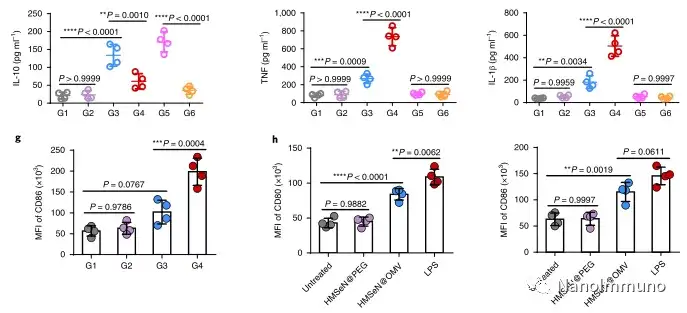
In this study, HMSen is rapidly degraded in both the oxidative tumor microenvironment and the bioreductive intracellular environment, and induces the explosive release of Annexin A5 on demand. The released annexin A5 prevents the exposure of phosphatidylserine on dying tumor cells, thereby preventing macrophages from recognizing it, and shifting immunosuppressive cell apoptosis toward immunostimulatory secondary necrosis, thereby Turn the tumor into an antigen library that can trigger a specific and powerful immune response. After systemic administration, HMSen-ANX5@HOMV caused inflammation in the tumor microenvironment, enhanced the host activation of DC, triggered a strong cytotoxic T cell immune response, and induced immune memory.
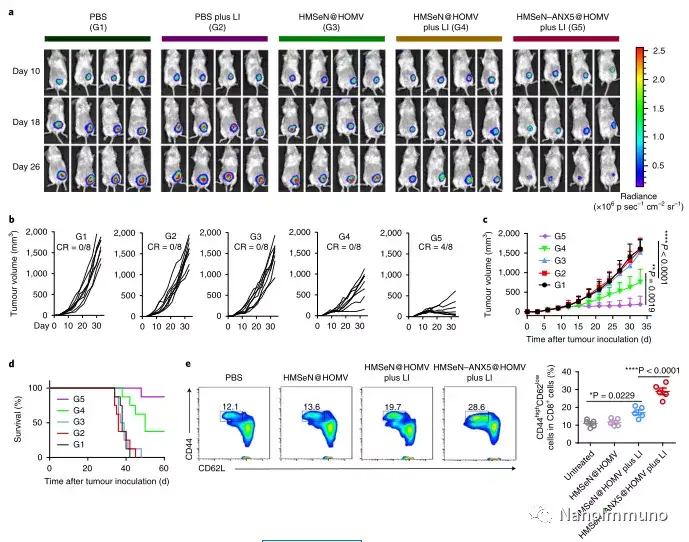
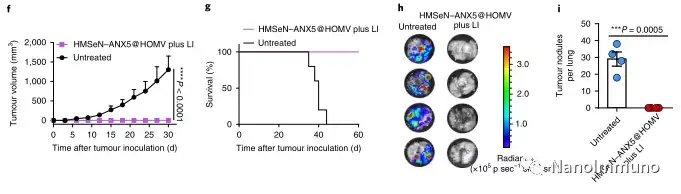
Most traditional tumor vaccines containing one or several tumor antigens cannot overcome tumor heterogeneity. Therefore, the antigen may not be completely related to a specific tumor. In the method described here, the primary tumor itself acts as an in situ antigen reservoir, which contains all tumor-associated antigen epitopes and injury-related molecular patterns required to initiate a powerful anti-tumor immune response. Another benefit is that this approach to in situ therapeutic vaccines avoids the cumbersome in vitro tumor antigen production. Synthetic amorphous silica is generally regarded as a safe material by the US Food and Drug Administration. Selenium is an important component of antioxidant enzymes in the human body and contributes to potential clinical transformation. In summary, given that most current cancer treatment methods can induce apoptosis, in situ therapeutic vaccine methods can provide a powerful and direct general method for the development of personalized in situ tumor vaccines.

Original link:
https://doi.org/10.1038/s41551-020-0599-5
NanoImmuno
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
https://doi.org/10.1038/s41551-020-0599-5
NanoImmuno
This information is sourced from the Internet for academic exchanges. If there is any infringement, please contact us to delete it immediately
18915694570
Previous: NML review | propertie


