Synergistic enhanced electrocatalytic hydrogen evolution of highly dispersed Ru nanoparticles on boron-doped Ti3C2Tx MXene nanosheets

1. Article overview
Two-dimensional layered materials have attracted more and more attention as low-cost support materials for the development of active catalysts for hydrogen evolution reactions. In addition, the atomically thin Ti3C2Tx (MXene) nanosheets have surface termination groups (Tx: F, O, OH), which are active sites for effective functionalization. In this work, heteroatom (boron)-doped Ti3C2Tx (MXene) nanosheets were developed into an efficient solid support to support ultra-small ruthenium (Ru) nanoparticles for electrocatalytic HER. Quantum mechanics first-principles calculations and electrochemical tests show that doping B on two-dimensional MXene nanosheets can greatly improve the adsorption kinetics of intermediate H*, reduce the charge transfer resistance to HER, and thus improve the reactivity and reactivity of active sites. Good electrode kinetics. Importantly, the newly designed electrocatalyst based on Ru nanoparticles supported on b-doped MXene (Ru@B Ti3C2Tx) nanosheets exhibited significant catalytic activity with overpotentials of 62.9 and 276.9 mV, respectively, which can drive 10 and 100 mA cm-2 HER, and has good cycle stability. In addition, through theoretical calculations, the Gibbs free energy (ΔGH* = 0.002 eV) of Ru@B Ti3C2Tx is close to zero. This work introduces a simple strategy to functionalize MXene as an effective solid support for electrocatalysts.
Two, graphic guide
Two-dimensional layered materials have attracted more and more attention as low-cost support materials for the development of active catalysts for hydrogen evolution reactions. In addition, the atomically thin Ti3C2Tx (MXene) nanosheets have surface termination groups (Tx: F, O, OH), which are active sites for effective functionalization. In this work, heteroatom (boron)-doped Ti3C2Tx (MXene) nanosheets were developed into an efficient solid support to support ultra-small ruthenium (Ru) nanoparticles for electrocatalytic HER. Quantum mechanics first-principles calculations and electrochemical tests show that doping B on two-dimensional MXene nanosheets can greatly improve the adsorption kinetics of intermediate H*, reduce the charge transfer resistance to HER, and thus improve the reactivity and reactivity of active sites. Good electrode kinetics. Importantly, the newly designed electrocatalyst based on Ru nanoparticles supported on b-doped MXene (Ru@B Ti3C2Tx) nanosheets exhibited significant catalytic activity with overpotentials of 62.9 and 276.9 mV, respectively, which can drive 10 and 100 mA cm-2 HER, and has good cycle stability. In addition, through theoretical calculations, the Gibbs free energy (ΔGH* = 0.002 eV) of Ru@B Ti3C2Tx is close to zero. This work introduces a simple strategy to functionalize MXene as an effective solid support for electrocatalysts.
Two, graphic guide
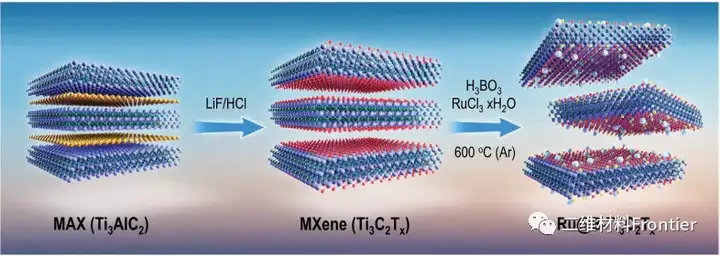
Figure 1. Synthetic schematic diagram of Ti3C2Tx and Ru@B-Ti3C2Tx.
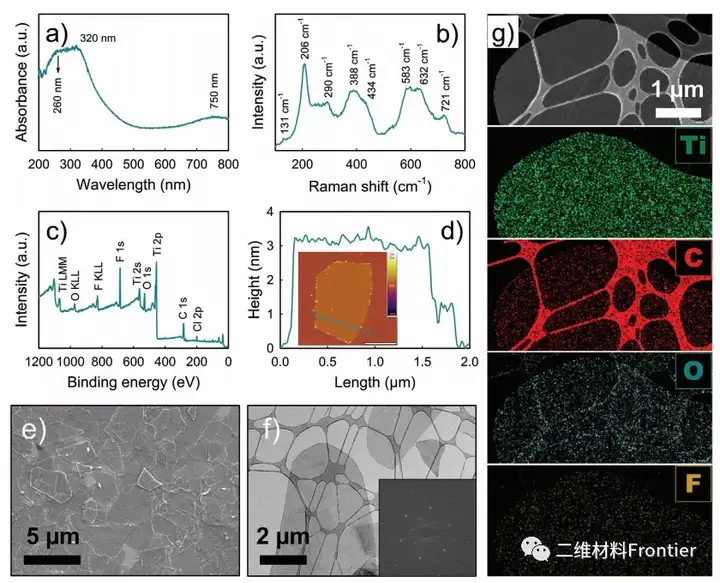
Figure 2. a) UV-Vis spectrum, b) Raman spectrum, c) XPS measurement scan, d) AFM image (scale bar: 1 μm) and corresponding height measurement, e) SEM image, f) TEM image and corresponding SAED pattern , G) HAADF-STEM image and corresponding EDX element (Ti, C, O, F) mapping image.
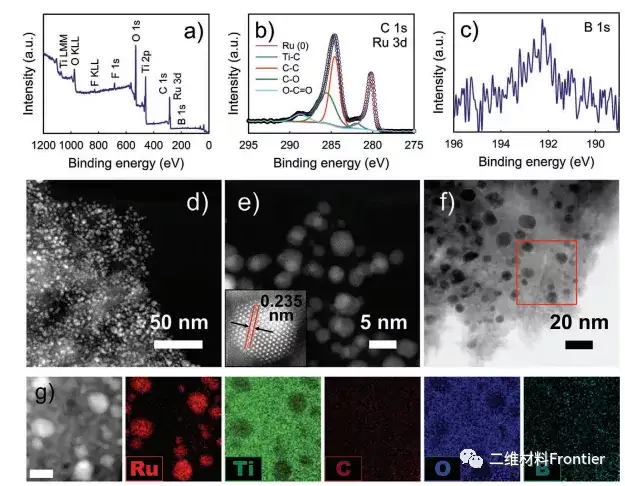
Figure 3. a) XPS measurement scan, b) high resolution C 1s and Ru three-dimensional spectrum, C) b 1s spectrum, d) TEM image of Ru@B Ti3C2Tx. g) The HAADF-STEM image of Ru@B Ti3C2Tx (scale bar: 10 nm) and the corresponding EDX element mapping image.
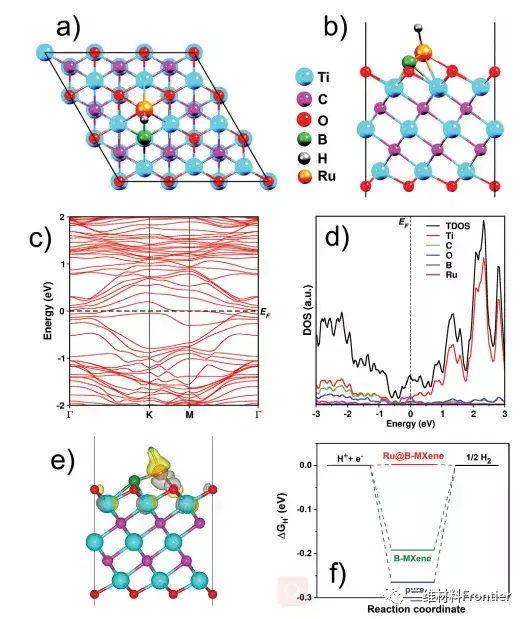
Figure 4. a) Top view and b) side view of Ru@B–Ti3C2Tx. c) The electronic band structure of Ru@B-MXene and d) the density of states. e) The charge difference of hydrogen adsorption on Ru@B-MXene. f) Calculated Gibbs free energy (ΔGH*) diagrams of Ti3C2Tx (pure MXene), B-Ti3C2Tx (B-MXene) and Ru@B-Ti3C2Tx under equilibrium potential. Third, the full text summary
In summary, we have successfully developed heteroatom (B)-doped Ti3C2Tx (MXene) nanosheets as solid supports supporting ruthenium nanoparticles for efficient electrocatalytic hydrogen production. In addition to using comprehensive experimental psychological analysis, we also used DFT calculations to explore the catalytic dynamics of our heteroatom-doped two-dimensional MXene nanosheets. Experimental research and theoretical calculations show that B doping significantly improves the adsorption kinetics of intermediate H* and reduces the charge transfer resistance of two-dimensional MXene nanosheets to HER. In fact, our newly designed catalyst, that is, Ru nanoparticles anchored B-doped MXene nanosheets (Ru@B Ti3C2Tx), achieves excellent catalytic activity, with low overpotentials of 62.9 and 276.9 mV, respectively, driving 10 and 100 mA The HER of cm-2 showed excellent stability during 1000 consecutive CV cycles. This work introduces a promising strategy for developing non-metallic B-doped electrocatalysts on two-dimensional MXene nanosheets and designing high-efficiency catalytic systems. Article link: https://doi.org/10.1002/smll.202102218.
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
In summary, we have successfully developed heteroatom (B)-doped Ti3C2Tx (MXene) nanosheets as solid supports supporting ruthenium nanoparticles for efficient electrocatalytic hydrogen production. In addition to using comprehensive experimental psychological analysis, we also used DFT calculations to explore the catalytic dynamics of our heteroatom-doped two-dimensional MXene nanosheets. Experimental research and theoretical calculations show that B doping significantly improves the adsorption kinetics of intermediate H* and reduces the charge transfer resistance of two-dimensional MXene nanosheets to HER. In fact, our newly designed catalyst, that is, Ru nanoparticles anchored B-doped MXene nanosheets (Ru@B Ti3C2Tx), achieves excellent catalytic activity, with low overpotentials of 62.9 and 276.9 mV, respectively, driving 10 and 100 mA The HER of cm-2 showed excellent stability during 1000 consecutive CV cycles. This work introduces a promising strategy for developing non-metallic B-doped electrocatalysts on two-dimensional MXene nanosheets and designing high-efficiency catalytic systems. Article link: https://doi.org/10.1002/smll.202102218.
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.
18915694570
Previous: Self-cleaning photocat


