Review: application of nano-biomaterials in the treatment and imaging of acute liver failure
In recent years, nano-biomaterials for the diagnosis and treatment of acute liver failure (ALF) have attracted more and more attention. The physiological characteristic of liver interception of most nanoparticles is the natural advantage of nano-biomaterials targeting liver. On the one hand, nanomaterials improve the therapeutic efficacy of ALF by improving the bioavailability of free drugs; on the other hand, nanomaterials increase the accumulation of therapeutic drugs in the liver to achieve more effective targeted therapy of the liver and even hepatocytes; in addition, stimulus-responsive, optical or magnetic nanomaterials show great potential in the multi-functional therapeutic platform for the treatment, diagnosis and imaging of ALF. Therefore, nano-biomaterials are of great significance and prospect in the application of ALF.

The highlight of this article.
1. The therapeutic mechanism and targeting strategy of nano-biomaterials in the treatment of acute liver failure were summarized.
2. This paper reviews the latest progress of a variety of nano-biomaterials in the treatment, diagnosis and imaging of acute liver failure.
Content introduction.
In this paper, Tao Yu, a researcher in the third affiliated Hospital of Sun Yat-sen University, discussed the mechanism of nanomaterials in the treatment of ALF; focused on the latest progress of various nanomaterials in ALF diagnosis, imaging and targeted therapy; summarized the targeting strategies of nanomaterials in ALF therapy; finally, the paper summarized and prospected the current challenges and research prospects in this field.
Guided reading of picture and text.
I. Mechanism of nanometer biomaterials in the treatment of acute liver failure.
Studies have shown that extensive hepatocyte necrosis, inflammatory storm, immune disorder and functional impairment are the main pathological features of acute liver failure. At present, nano-biomaterials have been widely used in the treatment of ALF in inhibiting inflammation and protecting hepatocytes. By loading anti-inflammatory or antioxidant drugs, nano-biomaterials can be targeted to deliver drugs to the site of liver injury or specific cells, so as to reduce liver injury caused by inflammatory and oxidative stress; it can also transmit the small interference RNA of pro-inflammatory cytokines to Kupffer cells, specifically inhibit the secretion of pro-inflammatory cytokines and inhibit large-scale inflammatory response. On the other hand, nanomaterial delivery system can also deliver drugs or genes with hepatoprotective function to the liver to reduce hepatocyte apoptosis or necrosis. In addition, nanotechnology can be used as an adjuvant therapy strategy for stem cell therapy to track the distribution and survival of transplanted cells. At the same time, nanoparticles containing cell growth factor can promote stem cell liver differentiation and liver regeneration through the continuous release of growth factor.
ii. Application of nano-drugs in the treatment of acute liver failure.
2.1 Nano biomaterials for the delivery of small molecular drugs.
Nano-biomaterials can be designed as a drug delivery system with good hydrophilicity, blood system stability and appropriate surface charge. therefore, nano-carriers have certain advantages in improving the water solubility of small molecular drugs and improving circulation stability. For example, Chen et al synthesized ROS-responsive polymer nanomaterials for melatonin delivery, providing sustained, on-demand, and targeted melatonin release from injured sites in mice with acute liver failure (figure 1). At the same time, nano-biomaterials have the natural advantage of passive accumulation in the liver, which can increase the enrichment of drugs in the liver; nano-biomaterials modified by specific cell receptor ligands or bioactive substances have the function of actively targeting specific cells in the liver, so as to achieve drug targeted therapy.
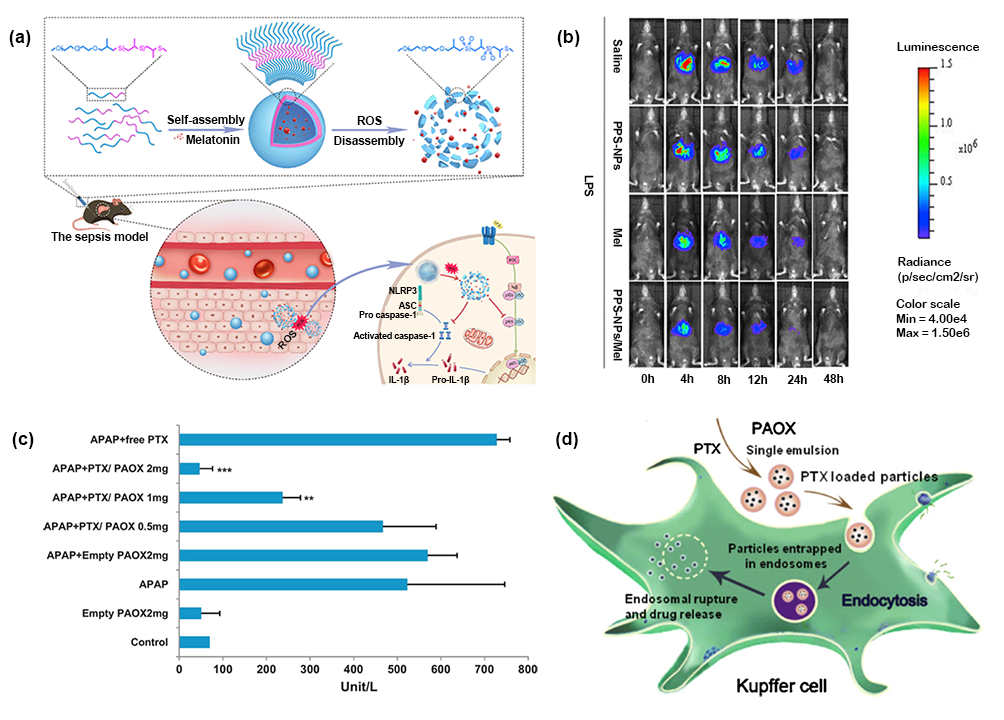
Figure 1. (a) A schematic diagram of melatonin delivery and anti-inflammatory mechanisms. (B) bioluminescence imaging of dynamic NF- kappa B activation in mouse liver after LPS stimulation. (C) the ALT level of each treatment group in acetaminophen (APAP)-induced ALF mice. (d) schematic diagram of intracellular drug delivery containing PAOX particles of PTX.
2.2 Nano biomaterials for delivery of gene therapy drugs.
Current studies have shown that nanomaterials such as cationic polymers and liposomes have been widely used as gene delivery systems for ALF therapy because of nucleic acid protection and excellent endosome / lysosome escape ability. The delivery of inflammation-related TNF- α siRNA, NF- κ B decoy, hepatocyte apoptosis-related Fas siRNA, hepatocyte regeneration-related IL-22 gene and microRNA122 related to stem cell differentiation into mature hepatocytes are all used in the treatment of ALF. For example, the He project combined helical peptide hybrid nanoparticles with transmembrane function for the effective delivery of TNF- α siRNA in acute liver failure, achieving 90% TNF- α knockdown in macrophage RAW 264.7 cells and ALF mouse models (figure 2).
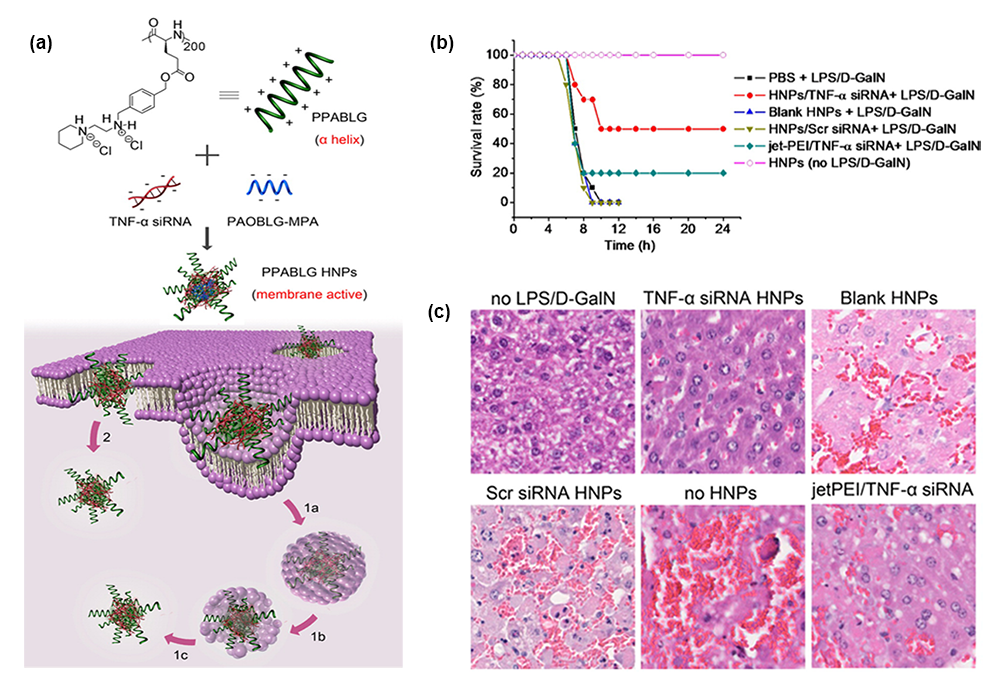
Figure 2. (a) schematic diagram of the synthesis, internalization and endosome escape of hybrid nanoparticles carried by TNF- α siRNA. (B) the survival rate of mice in different treatment groups after LPS/D-GalN stimulation. (C) HE staining of ALF mouse liver induced by LPS/D-GalN.
2.3 Nano-biomaterials for delivering cytokines.
Based on the therapeutic effect of stem cell secretory factor and stem cell differentiation into mature hepatocytes on ALF, nano-biomaterials have certain advantages in combining stem cells for better treatment of ALF. On the one hand, the secretory factors of stem cells can be collected and concentrated in nanoparticles, so that a high concentration of secretory factors can be delivered to the liver (figure 3). On the other hand, the differentiation of stem cells into mature hepatocytes requires long-term induction of growth factors, high cost and long cycle. Therefore, it is necessary to study nano-biomaterials for the effective and continuous release of growth factors. Wang et al assembled hepatocyte growth factor (HGF), acidic fibroblast growth factor (aFGF) and activin An into polyethyleneimine (PEI) modified SiO cells NPs to form a continuous delivery system of growth factors and shorten the time for mouse embryonic stem cells to differentiate into mature hepatocytes.
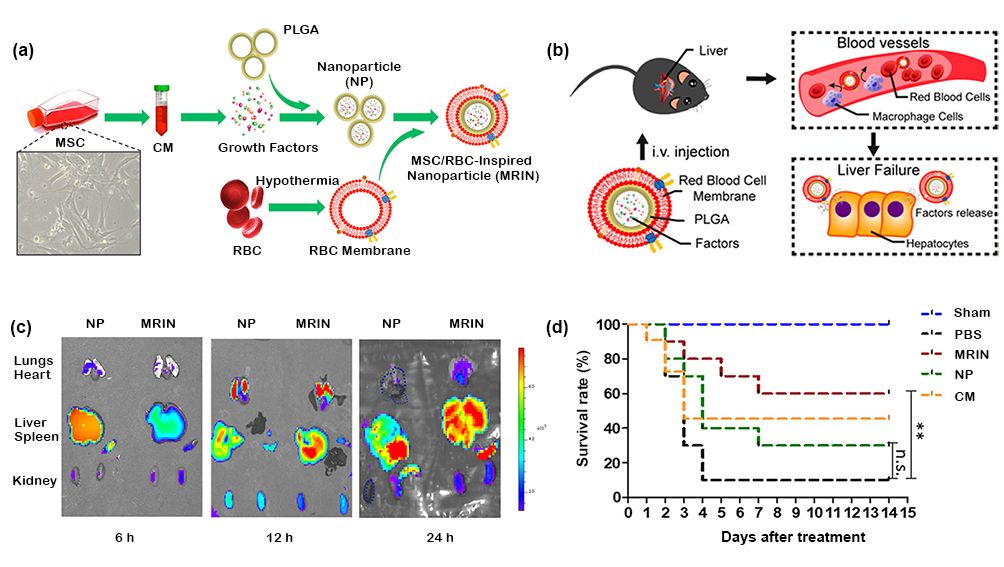
Figure 3. (a) schematic diagram of MRIN synthesis process. (B) the in vivo and intracellular kinetics of MRIN during ALF treatment. (C) the distribution of nanoparticles in ALF mouse model. (d) the survival rate of ALF mice induced by CCl challenge.
2.4 Nano-enzyme in the treatment of acute liver failure.
Nano-enzyme is a kind of nano-material with enzyme-like properties. SOD and catalase mimics can scavenge ROS and play a role in anti-inflammatory therapy. Catalase mimics can scavenge H2O2 in APAP-related ALF and reduce the level of ALT in serum. Therefore, nano-enzyme has become an effective way to treat ALF.
Mechanism of targeting liver by nano-drugs in the treatment of acute liver failure with III.
The delivery methods of nanoparticles are usually divided into passive targeting and active targeting. Passive targeting means that the physiological and anatomical characteristics of the liver allow nanoparticles with specific size or surface characteristics to accumulate in the liver. Active targeting uses specific ligands to bind to cell receptors to achieve specific drug / gene delivery.
3.1 passive targeting.
The properties of nanoparticles (size, hydrophobicity and surface charge) are the most basic factors that determine the ability of passive targeting cells or tissues. Proper adjustment of physical and chemical properties can make nano-drugs enter into hepatocytes in a passive targeting way. In the liver, since the pores of endothelial cells in the normal hepatic sinusoids are about 50-200 nm, nanoparticles less than 200 nm in diameter can pass through the hepatic sinusoids and thus reach hepatocytes or hepatic stellate cells, while larger particles are more likely to be swallowed by Kupffer cells. Nanoparticles with hydrophilic surfaces are almost not absorbed by the mononuclear phagocytic system, which is conducive to accumulation in the liver. The surface charge of nanomaterials needs to strike a balance between cell internalization ability and serum stability, maintain membrane affinity with appropriate positive charge, and avoid the adhesion of serum proteins in blood.Untargeted or unmodified nanomaterials are mainly absorbed by non-parenchymal cells in the liver, such as Kupffer cells, sinusoidal endothelial cells and hepatic stellate cells, and are mainly captured by Kupffer cells.
3.2 active targeting.
Nanoparticles modified with cell ligands are usually recognized by specific receptors of specific cells or diseases. In the treatment of ALF, targeting macrophages (Kupffer cells) and hepatocytes are the most important treatment strategies (figure 4). Desialoglycoprotein receptor (ASGP-R) is a specific receptor expressed on the membrane of hepatocytes. ASGP-R has an innate affinity for galactose and N-acetyl-galactosamine residues. Its common target ligands are galactose, galactoside, aminogalactose, lactic acid, desialylferritin and sterol glycosides, which are usually used to modify nanomaterials for active targeting. Galactose and lactic acid ligands are the most commonly used targeting methods in ALF targeting therapy. In addition, mannose and fucose receptors were overexpressed on the surface of Kupffer cells. Zhang et al used carboxylated mannan-modified nanocomposites to bind to mannose receptors on macrophages to increase the internalization rate of TNF- α siRNA in Kupffer cells.
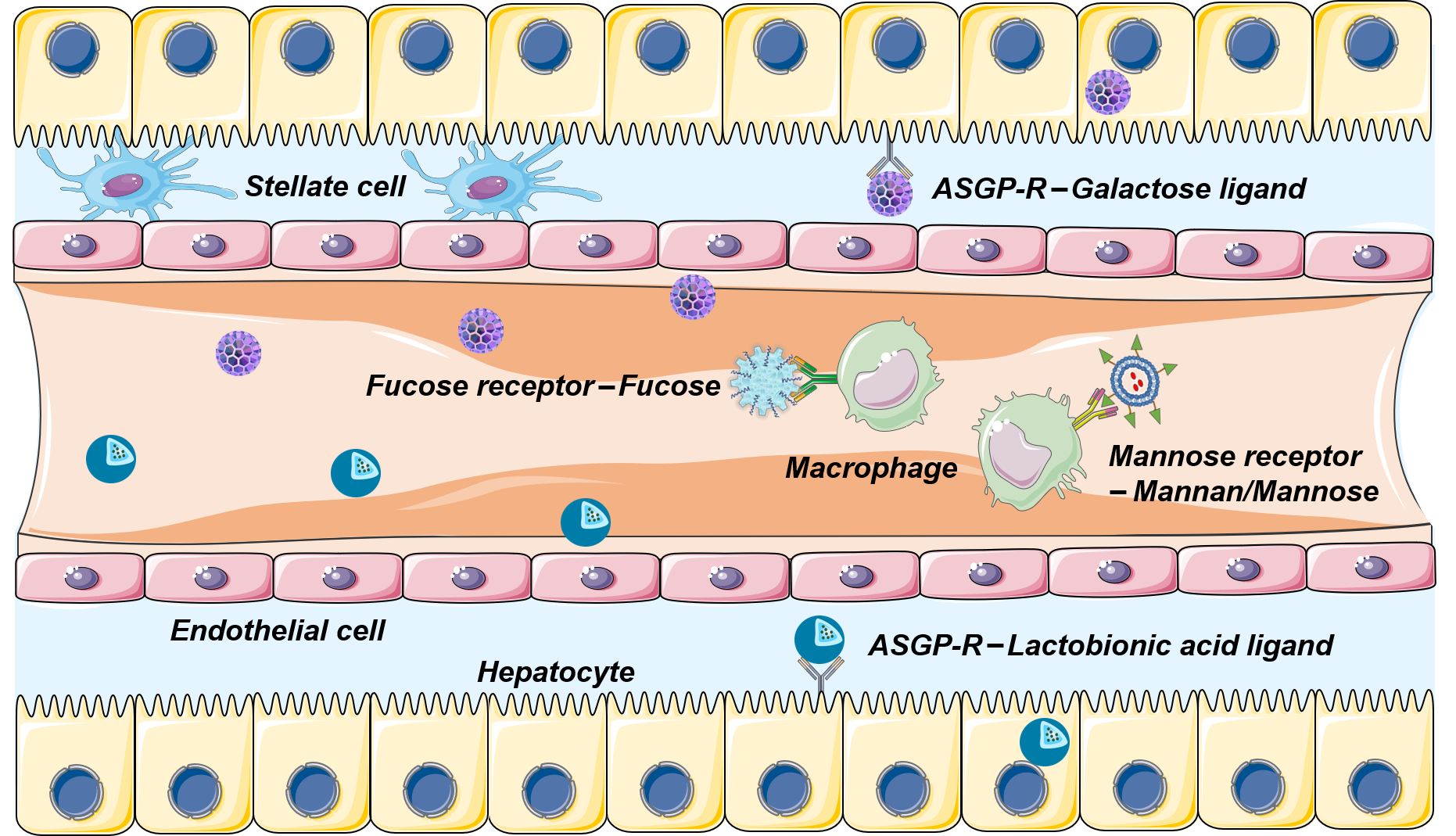
Figure 4. Schematic diagram of active targeting of hepatocytes and macrophages in ALF therapy.
Application of IV nano-biomaterials in imaging of acute liver failure.
Nanomaterials with optical or magnetic properties can be used as contrast agents for cells or tissues in ultrasound, X-ray, CT or MRI imaging. Nanomaterials with fluorescence imaging functions, such as quantum dots (QDs), are used to track stem cells transplanted in the liver. Not only the distribution and translocation of stem cells can be observed, but also the living stem cells can be labeled. Magnetic nanomaterials, such as hydroxyapatite-Fe3O4 nanomaterials synthesized by Xu et al, increase the noise ratio of damaged liver tissue from 3.71 to 5.39, which is helpful for the diagnosis and grading of acute liver injury. Contrast nanomaterials can also be loaded with different drugs and become multi-functional nanomaterials for both treatment and diagnosis. The nanocomposites composed of porous silicon, gold nanoparticles and surface-coated acetal glucan have both the function of therapeutic drug delivery and the imaging function of gold nanoparticles to enhance CT imaging signals. Because the nanocomposites are mainly absorbed by macrophages gathered in the damaged area, the specificity enhances the local signal in the diseased area of the liver (figure 5).Nanomaterials with pH or H2O2 response can not only target the anti-inflammatory treatment of the inflammatory site, but also produce CO2 particles in the injured site and enhance the ultrasonic imaging signal.
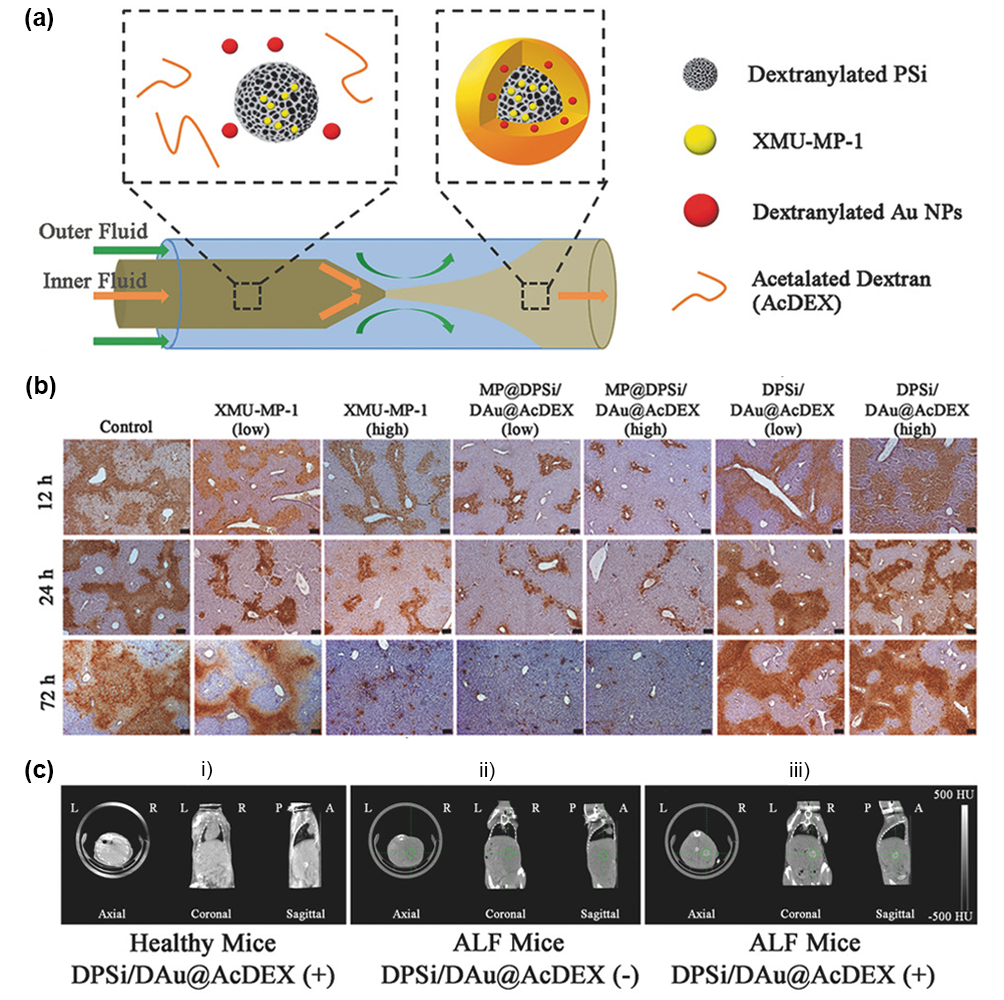
Figure 5. (a) Nanocomposites were prepared by microfluidic system. (B) TUNEL staining in liver tissue of mice in different treatment groups after APAP stimulation. (C) CT imaging of different groups of mice.
V summary and prospect.
This paper summarizes the existing research achievements and progress of nanomaterials in the treatment of ALF. Nanomaterials have unique advantages in ALF therapy because of their inherent characteristics. On the one hand, as a delivery system, nano-biomaterials can passively or actively target drugs to accumulate in liver injury sites or cells, improve the bioavailability, water solubility and half-life of free drugs, and protect the activity of nucleic acids and growth factors. On the other hand, magnetic or optical nanomaterials have imaging function and can be used for cell labeling; combined with the delivery of therapeutic drugs, a multi-functional therapeutic drug platform can be constructed. Therefore, nano-biomaterials have a broad prospect in the treatment of ALF.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.
18915694570
Previous: Advanced Functional Ma


