University of Maryland: a simple way to synthesize protective skin around any hydrogel
[background].
In nature, various structures such as fruits and vegetables have a water-rich core that is covered by a hydrophobic layer, that is, their skin. The skin forms a barrier to prevent chemicals from the external environment from entering the core; at the same time, the epidermis ensures that the water in the core is preserved and will not be lost by evaporation.At present, for many applications involving hydrogels, especially in the fields of soft robots or bioelectronic interfaces, it will be beneficial if the gel can be wrapped in skin-like materials. However, the formation of such skin around the gel has proved to be challenging because the skin needs to be a hydrophobic material with different chemical properties from the core of the hydrophilic gel.
[abstract].
Recently, a team of professors at the University of Maryland, Srinivasa R. Raghavan, came up with a simple solution to this problem, which allows any hydrogel of any composition and geometry to be wrapped in a thin and transparent skin. The synthesis technique involves polymerization from the inside out, in which one component of the polymerization (initiator) exists only in the core of the gel, while the other components (monomers) exist only in the external medium. Therefore, the thin polymer layer (about 10-100 μ m thick) grows outward from the core, and the whole process can be completed in a few minutes. The team showed that the presence of skin can prevent the gel from expanding in water and drying in air. Similarly, the hydrophilic solute in the core of the gel is completely prevented from leaking into the external solution by the skin.
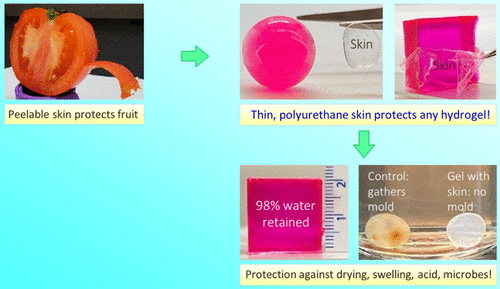
At the same time, it prevents irritating chemicals (such as acids, bases and chelating agents) or harmful microorganisms from entering the gel. The properties of the skin are adjustable, including its thickness and its mechanical properties. When the monomer used is ethyl carbamate diacrylate, the surface layer of the polyurethane is elastic, transparent and can be stripped from the core gel.In contrast, when polyethylene glycol dimethacrylate is used as a monomer, the epidermis is hard and brittle (glassy).The ability to easily grow skin around any given hydrogel may prove useful in many applications, such as in maintaining the electrical function of gel-based wires or circuit components. The related papers are published in ACS Appl under the title A Simple Way to Synthesize a Protective Skin around Any Hydrogel. Mater. Interfaces .
[picture and text analysis].
Hydrogels are water-swollen polymer networks with solid-like properties. They appear in different fields, including biomedicine (for example, as a scaffold for tissue engineering), pharmaceuticals (for example, as a matrix for drug delivery), and the food industry where various edible materials are in a hydrogel state. Recently, new applications of hydrogels have emerged in soft robots and biomedical devices that can be connected to the body. In many of these cases, the utility of hydrogels is limited by the tendency that their surfaces become dry (dehydrated) when exposed to ambient air. For example, consider the gelatin gel (Jell-O) cube, which is usually made as a dessert in the home. If you leave this gel on the table, it will significantly dry in less than a day and lose its texture and flavor. Similarly, figure 1 shows that the acrylamide (AAm) gel prepared as a 2cm cube in the laboratory shrank more than half of its original volume within 17 hours.
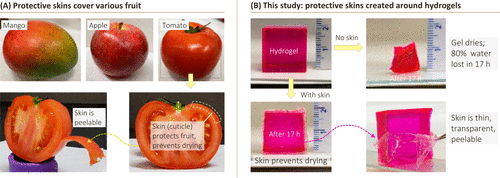
Figure 1. The natural inspiration for the methods outlined in this study. (a) examples of fruits with hydrophobic epidermis (stratum corneum), including mangoes, apples and tomatoes. Shows the cut part of the tomato, highlighting the skin, which is also shown to be peelable. (B) the cube-shaped hydrogel dried significantly after exposure to ambient air for 17 hours. However, if the same gel is wrapped in a thin hydrophobic surface, the water loss will be greatly reduced. The skin is thin, elastic, transparent and can be removed from the gel with tweezers.
For comparison, consider a variety of fruits or vegetables, with examples (mangoes, oranges, and tomatoes) shown in figure 1. These are soft materials, which contain a lot of water. Special attention is paid to tomatoes (figure 1A), of which more than 60% is water (similar to many gels prepared in the laboratory). Figure 1B shows the typical epidermis around the AAm gel cube: note that the epidermis is transparent and can be peeled off from the core. This skin inhibits the transport or entry of the gel: specifically, the gel stops expanding when it is placed in water, while the gel exposed to air loses very little water. Similarly, solutes (such as model drugs or proteins) can be stored in the gel core for a long time, while irritating chemicals (such as acids) or pollutants (such as microorganisms) in the external solution are prevented from entering the gel.
Skin synthesis.
The process of covering the hydrogel with hydrophobic skin is shown in figure 2. The spherical gel of alginate (4 mm in diameter) is the starting point. The gel is made by dripping 2% sodium alginate solution into 0.5 M calcium chloride using a syringe. The alginate chain in a given droplet is cross-linked into a network by divalent Ca2+ ions to form a transparent spherical gel with a diameter of about 4mm. The team added 0.05% acid red dye to provide visual clarity of the gel. In order to form skin around the gel, it was first incubated in an aqueous solution containing 0.5% water-soluble photoinitiator (Irgacure 2959, a benzophenone derivative, about 1% water soluble). After 5 minutes, the initiator-loaded gel (Photo A1) is placed in the liquid monomer, in this case oligocarbamate diacrylate (UDA).
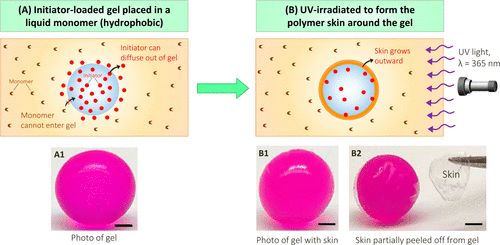
Figure 2. The process of synthesizing skin around a hydrogel. (a) the hydrogel loaded with initiator (photo A1) is placed in a monomer (UDA) solution. Choose the initiator that is soluble in the monomer, but the monomer is insoluble in water. Therefore, the initiator can diffuse out of the gel, but the monomer cannot enter the gel. (B) the skin (UDA polymer) grows outward after exposure to 365 nm UV. Photos B1 and B2 show that the skin is thin and transparent. To show it clearly, use tweezers to peel off part of the skin. Scale: 1 mm.
Skin appearance, thickness and microstructure.
Figure 3A shows the skin formed under two different UV exposure times. These experiments were carried out with 4 mm alginate gel containing 0.5% initiator. After UV irradiation for 10 minutes, 60 μ m skin was detected around the gel (figure A1). Increasing the UV exposure time to 90 minutes will cause the skin to become 140 microns thicker (image A2). Scanning electron microscope (SEM) images further confirm the uniformity, as shown in figure 3B. Here, after 30 minutes of ultraviolet radiation, skin is formed around the alginate gel (3 mm in diameter).
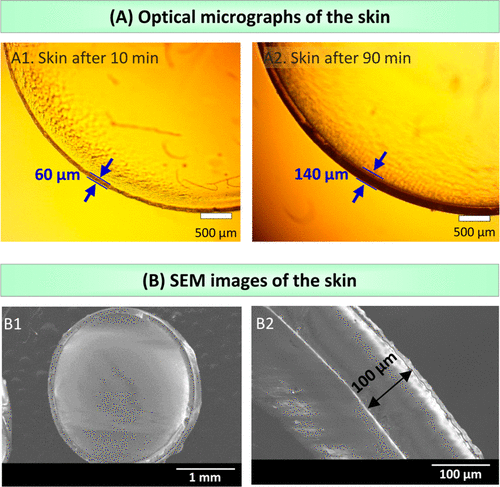
Figure 3. Observe the skin by microscope.
The skin around various gels.
The teams technique of forming skin around the hydrogel is simple and convenient. It can be used to wrap any hydrogel of any composition or shape or mechanical properties. To prove this, we prepared alginate, AAm and polyethylene glycol diacrylate (PEGDA) gels and wrapped them all in the polyurethane epidermis (figure 4).
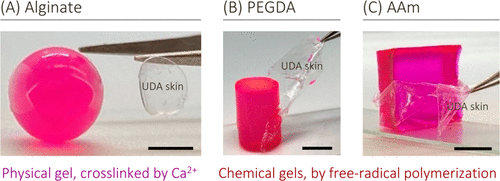
Figure 4. Different hydrogels with different chemical properties and shapes surround the skin. Spherical alginate gel, cylindrical PEGDA gel and cubic AAm gel all showed thin UDA skin. Use tweezers to peel off parts of the skin to indicate their presence.
Soft and hard skin.
The experiment proved the difference of mechanical properties between PEGDMA and UDA skin. If you place the PEGDMA-covered alginate gel on the workbench and hit it with a hammer, the skin will break into pieces while the core gel remains intact (figure 5 illustration).
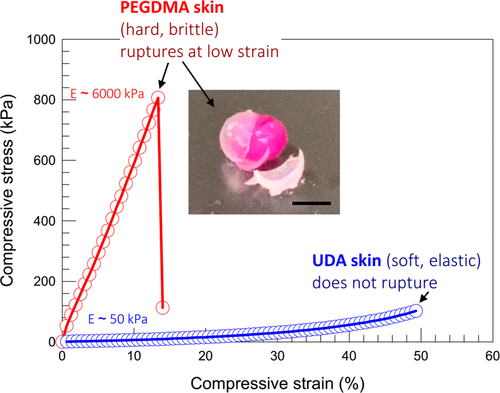
Figure 5. Compare the mechanical properties of hard skin and soft skin. The compressive stress and strain of alginate gel covered with UDA and PEGDMA.
The skin prevents the gel from drying in the air and expanding in water.
Next, the team showed that the presence of the skin made the hydrogel resistant to dehydration and expansion in water (figure 6). Figure 6A compares the drying of skin-covered gel and naked gel in ambient air, both of which are cubes of 2 cm in length. The team then studied the counterpart of the above phenomenon, that is, whether the skin could inhibit swelling (water intake) when the gel was placed in a water bath (figure 6B).
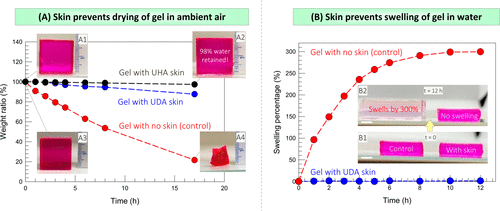
Figure 6. Test the ability of the skin to prevent water from / into the gel.
Skin regulates solute transport outside the gel.
Figure 7A shows the relationship between cumulative dye release (as a percentage of the total) and time. In less than 1 hour, 80% of the dyes were released from the naked gel into the external solution, while in 4 hours, almost all dyes were released. This is expected because the size of the dye molecule is smaller than nanometer, which is much smaller than the mesh size of alginate gel. Therefore, the dye diffuses easily and the rapid release of small solutes from the gel is well documented in the literature. However, on the contrary, figure 7A shows that even after a day, the dye will never be released from the skin-covered alginate gel.
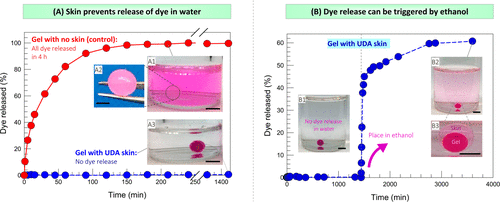
Figure 7. Test the release of solute from the skin-covered gel in water and ethanol.
The skin blocks transport to the gel.
Figure 8A shows an example in which a gel loaded with a pH indicator dye (methyl red) is placed in a strong acid (1m HCl). Figure 8B shows that alginate gels stored in water at room temperature will become moldy within 3 months (photo B2). In contrast, alginate gels covered with thin skin of UDA and placed in the same vial did not produce any mold at the same time. Therefore, the skin can protect alginate gel from microbes.
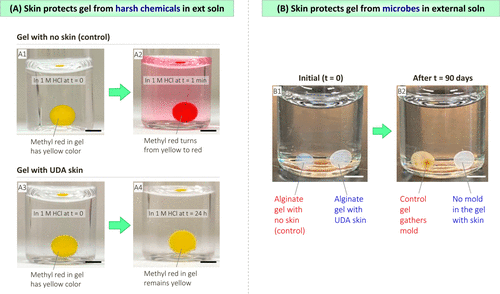
Figure 8. Test the ability of the skin to protect the gel from irritating chemicals and microorganisms.
Skin-covered gel as an electrical conductor.
Today, gels are being evaluated in electronic sensors, where they will come into contact with the skin (so the gel will sense analytes present in the body). The gel is also used as an electrolyte in flexible batteries or as an actuator in soft robots. In such future applications, gels that can resist dehydration (when covered by skin) may be beneficial. To explore this, the team conducted a simple experiment in which a cylindrical gel was used as a conduit or wire in the circuit (figure 9).
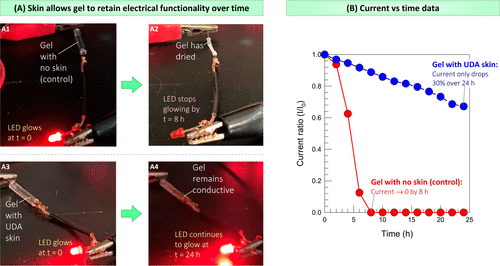
Figure 9. Test the skin-covered gel as a catheter in the circuit. The DC power supply is connected to the LED through a cylindrical gel, which is used as an ionic conductor on part of the circuit.
[summary].
The team designed a simple technique to encapsulate any hydrogel in hydrophobic skin. This technique is an inside-out polymerization in which the gel loaded with the initiator is placed in an organic (liquid) monomer. The team chooses initiators that are soluble in water-based gels and monomers, which are hydrophobic and therefore insoluble in gels. After being irradiated with ultraviolet light, a polymer surface layer will grow around the gel. The whole process is convenient, fast and carried out under mild conditions. Skin can be formed around gels of various geometric shapes, and this process is equally effective for gels formed by physical or covalent bonds. The thickness of the skin can be easily controlled by ultraviolet exposure time; typically, the team forms about 100 microns of skin around a 3-5 mm gel (that is, the skin is 2-3% of the gel size) after 10 minutes of exposure. The selection of organic monomers determines the mechanical properties of the skin. Using UDA as a monomer, the surface layer of polyurethane is transparent and elastic (soft and elastic) and can be peeled off from the core gel using tweezers. When PEGDMA is used as a monomer, a hard and brittle epidermis is formed around the gel core, which can be cracked by hitting it with a hammer.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.
18915694570
Previous: ACS NANO: multi-respon


