Preparation and excellent performance of flexible large spacing MXene membrane electrode by Natural sedimentation method
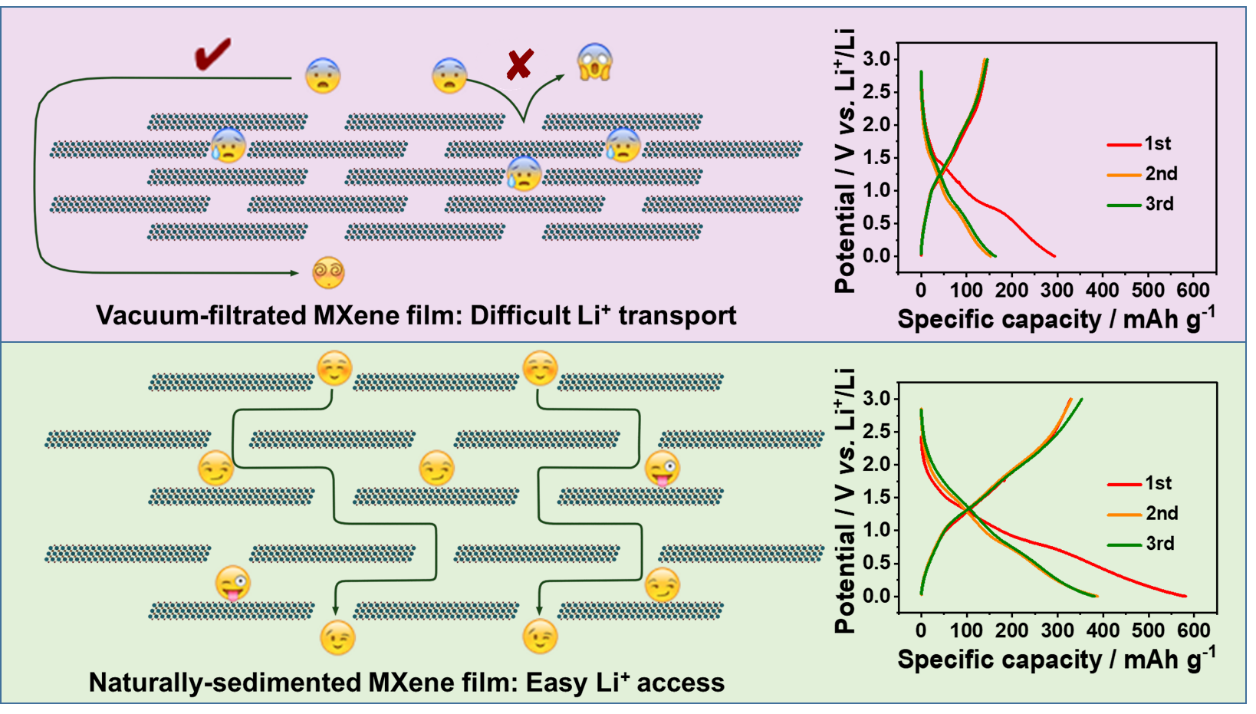
Enhanced Ionic Accessibility of Flexible MXene Electrodes Produced by Natural Sedimentation.
Ning Sun, Zhaoruxin Guan, Qizhen Zhu, Babak Anasori, Yury Gogotsi*, Bin Xu*.
Nano-Micro Lett. (2020) 12:89.
Https://doi.org/10.1007/s40820-020-00426-0.
The highlight of this article.
1. A simple and efficient strategy for preparing flexible MXene membrane electrode: natural sedimentation method is proposed.
2. The prepared MXene membrane electrode has a large layer spacing, which can effectively improve the ion transport between layers and improve the electrochemical performance of the material.
3. Compared with the MXene membrane electrode prepared by traditional vacuum filtration method, the MXene membrane electrode prepared by natural sedimentation method shows high lithium storage capacity and good cycle and rate performance.
Content introduction.
Professor Xu Bin of Beijing University of Chemical Technology and Professor Yury Gogotsi of Drexel University in the United States proposed a new strategy for the preparation of flexible self-supporting MXene membrane electrode by natural sedimentation to solve the problem that the close stacking of nano-layers in MXene membrane electrode seriously affects the electrochemical performance. Compared with the MXene membrane electrode prepared by traditional vacuum filtration method, the interlayer structure of MXene electrode prepared by natural sedimentation method is looser, the interlayer spacing is larger, and the storage and transport performance of ions between layers are significantly improved. When the flexible self-supporting Ti3C2Tx MXene membrane electrode prepared by natural sedimentation method was used as the negative electrode of lithium ion battery, its reversible capacity was up to 351mAh/g, which was much higher than that of MXene membrane electrode prepared by traditional vacuum filtration method (145mAh/g). At the same time, it also showed excellent cycle stability (no capacity attenuation after 1000 cycles) and rate performance.
Guided reading of picture and text.
Morphology and structure characterization of flexible self-supporting MXene membrane electrode.
The aqueous solution containing 10 mg MXene material was diluted to a solution with a concentration of 0.5,1 and 2 mol/L, respectively, and then placed in a filtration device equipped with Celgard 3501 membrane (diameter 4 cm) until the MXene membrane was dried, and the naturally settling MXene membrane (named Nat-0.5, Nat-1 and Nat-2, respectively) was obtained. The MXene membrane (Vac-0.5) obtained by traditional vacuum filtration and drying of MXene solution with 20 mL concentration of 0.5 mol/L was used as control. As shown in figure 1, the electrode prepared by natural sedimentation method shows good mechanical flexibility. At the same time, the structure of the electrode is looser than that of the vacuum filtered MXene electrode Vac-0.5, the thickness of the electrode increases from 3.31um of Vac-0.5 to 4.05um of Nat-0.5 (increased by ~ 22%), and the (002) peak in the XRD spectrum shifts to a low angle, indicating an increase in interlayer spacing (14.0612.20 vs. 14.7612.56). In the process of natural deposition, the smaller the concentration of MXene solution is, the thicker the electrode thickness of MXene thin film is, and the larger the interlayer spacing of (002) crystal plane is.
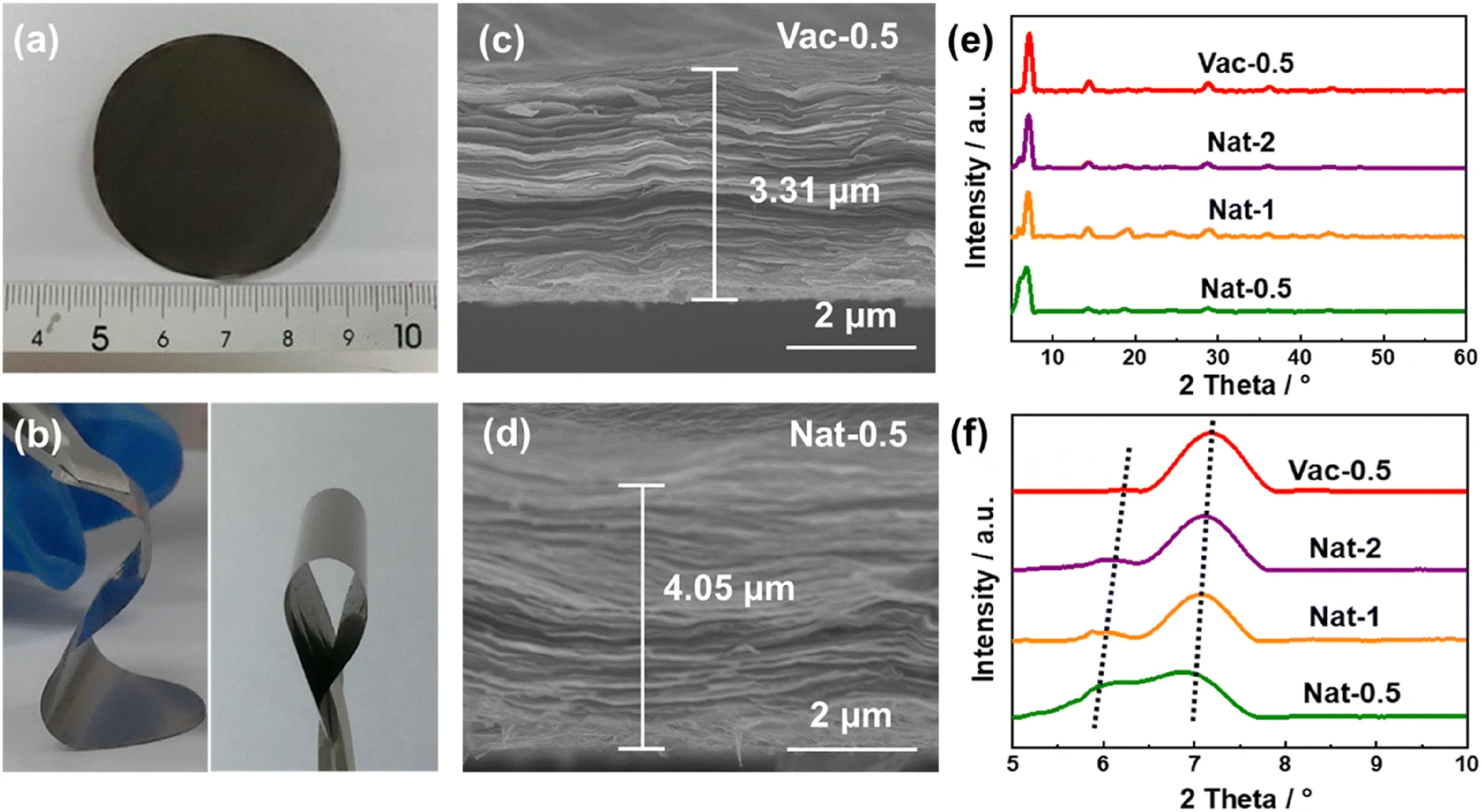
Figure 1.
(a) digital photographs of Nat-0.5; (c) cross-sectional SEM diagrams of Nat-0.5 and (d) Vac-0.5; and XRD spectra of Vac-0.5,Nat-2,Nat-1 and Nat-0.5.
Electrochemical Lithium Storage behavior of II MXene membrane electrode.
The vacuum filtration and natural settling MXene films were cut into wafers with a diameter of 10 mm and directly used as working electrodes to assemble lithium-ion batteries for electrochemical tests such as CV and galvanostatic charge-discharge. As shown in figure 2, the Vac-0.5 CV curve of the MXene electrode prepared by vacuum filtration has two obvious redox pairs at 1.54pm 2.10V and 0.71max 1.09 V, corresponding to the intercalation and detachment behavior of lithium ions between MXene layers with different layer spacing. However, the CV curve of Nat-0.5 prepared by natural sedimentation method is obviously different from that of vacuum filtered Vac-0.5, and the two groups of redox pairs tend to merge, because the increased interlayer spacing makes the intercalation of lithium ions change from a sequential process to a simultaneous process. In addition, the large interlayer spacing exposes more active sites and improves the storage and transport performance of lithium ion, so the MXene electrode prepared by natural deposition method shows high lithium storage capacity, in which the reversible capacity of Nat-0.5 is 351 mAh/g, which is much higher than that of Vac-0.5 (145 mAh/g).
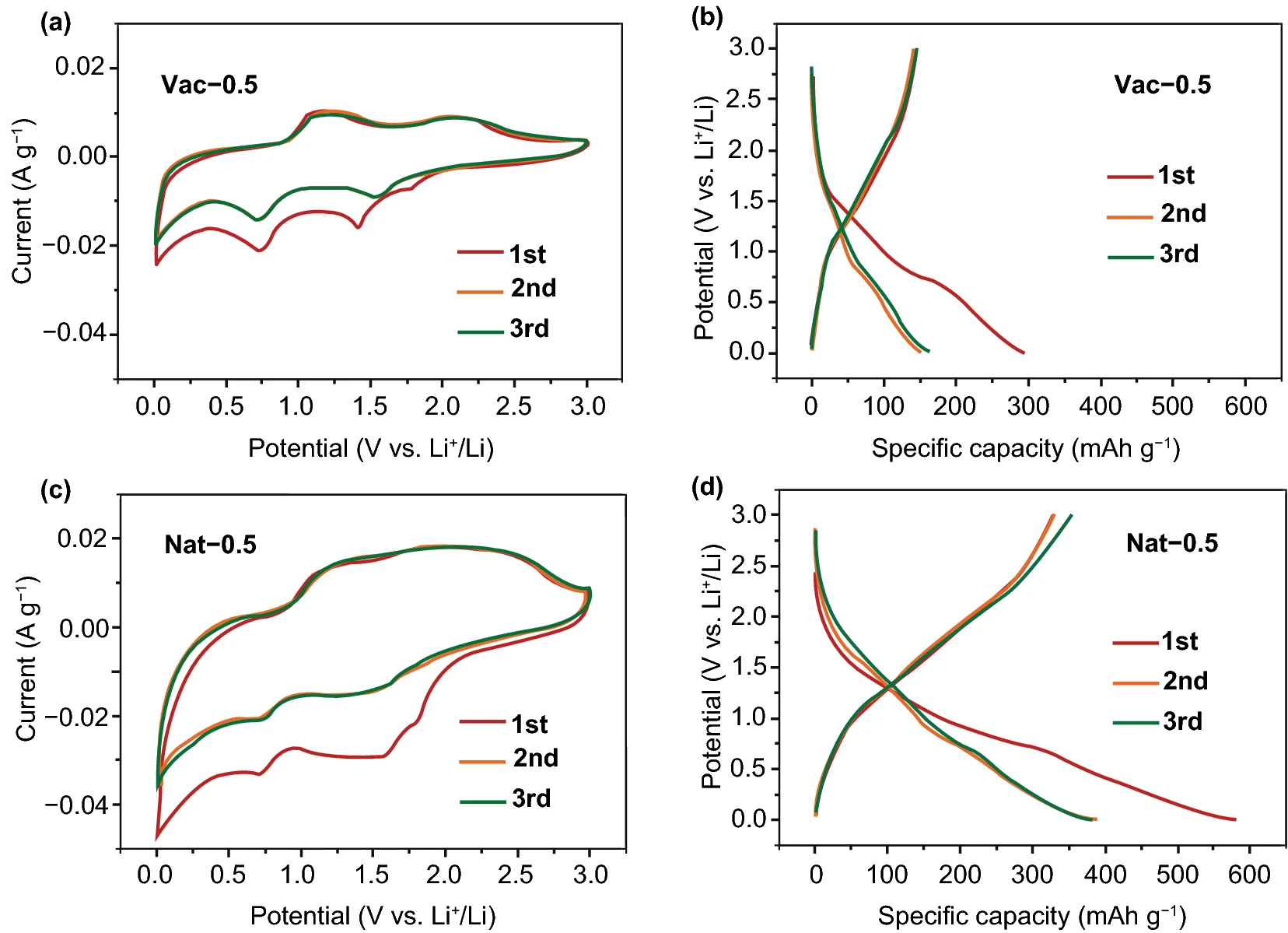
Figure 2.
The CV curve of Vac-0.5 and Nat-0.5 at the sweep rate of 0.1 mV/s and the charge-discharge curve at the current density of 30 mA/g.
Lithium storage mechanism of III MXene membrane electrode.
The morphology and structure of the discharged MXene electrode were observed and characterized, as shown in figure 3. Compared with the original electrode, the morphology of the Vac-0.5 and Nat-0.5 electrode did not change obviously, but the thickness of the electrode increased by 0.7 μ m and 0.08 μ m, respectively. Compared with Vac-0.5, the increased thickness of Nat-0.5 is negligible, indicating that the MXene electrode prepared by natural deposition has better structural stability. Accordingly, the (002) peak in the XRD spectrum shifts to a low angle in the discharge state, indicating that the interlayer structure of MXene is stretched and the layer spacing increases due to the intercalation of lithium ions.
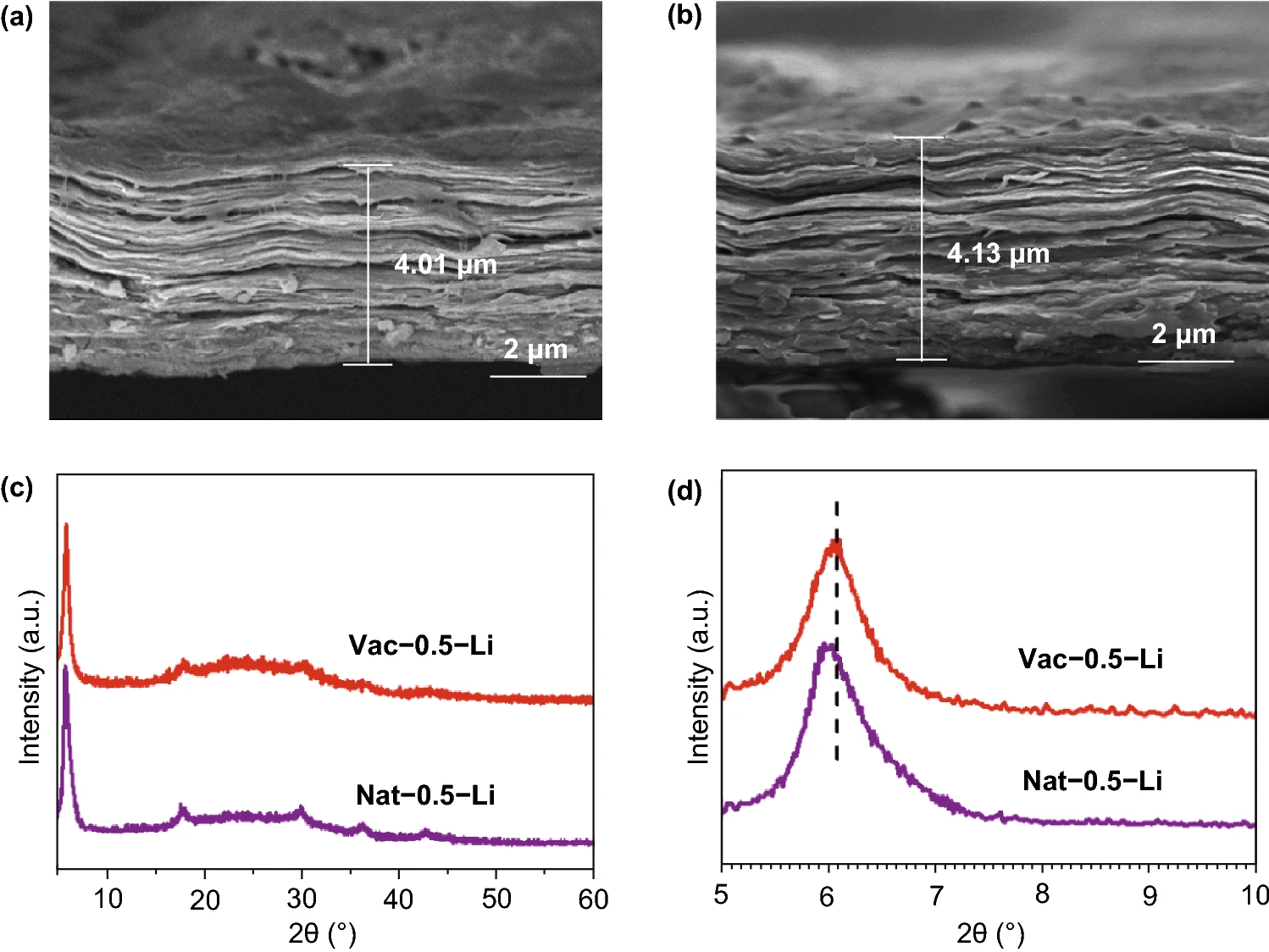
Figure 3.
(a) the cross-sectional SEM diagrams of Vac-0.5 and (b) Nat-0.5 after discharge, and the XRD spectra of Vac-0.5 and Nat-0.5 electrodes after discharge.
In order to further reveal the kinetic process of lithium storage of MXene electrode prepared by natural sedimentation method, the MXene electrode was tested by variable scanning rate CV (0.1 ~ 2 mV/s), as shown in figure 4.The charge storage mechanism of the electrode is analyzed by using the power law relation I = avb. According to the fitting calculation, the b value of Nat-0.5 is 0.854, indicating that the lithium storage process is controlled by surface reaction and diffusion behavior at the same time. Compared with 0.785 of Vac-0.5, the b value of Nat-0.5 is closer to 1, indicating that the large interlayer spacing and loose interlayer structure of Vac-0.5 increase the contribution of lithium storage controlled by surface non-diffusion process.In addition, with the increase of scanning speed, the proportion of non-diffusion control behavior increases gradually. When the scanning speed is 0.1 mV/s, the current contribution of non-diffusion control of Nat-0.5 is 54.0%. When the sweep speed is increased to 2 mV/s, the current contribution of non-diffusion control increases to 83.2%.
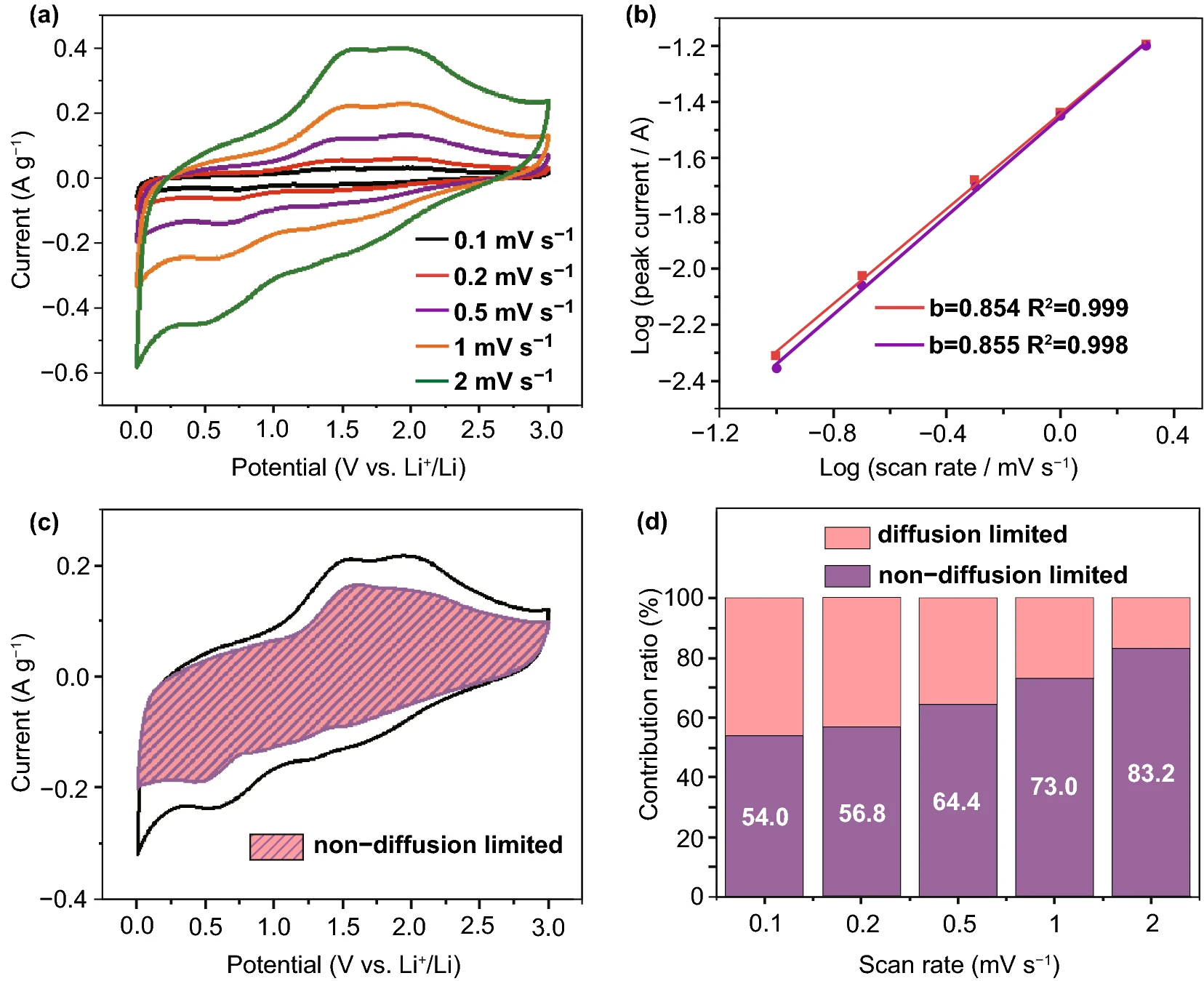
Figure 4.
(a) CV curve of Nat-0.5 at different scanning rates of 0.1: 2 mV/s; (b) log (v)-log (I) relationship based on Nat-0.5 oxidation peak fitting (red: oxidation peak of ~ 2.0V; purple: oxidation peak of ~ 1.5V); (c) proportion of non-diffusion control behavior in CV curve of Nat-0.5 at scanning rate of 1 mV/s (shaded part). (d) the current contribution of non-diffusion control behavior of Nat-0.5 at different scanning rates.
Electrochemical performance of IV MXene membrane electrode.
Fig. 5 compares the cycle and rate performance of vacuum filtration MXene electrode and natural sedimentation MXene electrode. The MXene electrodes prepared by the two methods showed good cycle stability. After 100 cycles at the current density of 50 mA/g, the reversible specific capacity of Nat-0.5 was stable at 266 mAh/g,Vac-0.5 and 104 mAh/g.Because the large interlayer spacing and loose interlayer structure are conducive to the rapid diffusion / transport of lithium ions, Nat-0.5 shows excellent rate performance. At 500 mA/g current density, the reversible specific capacity of Nat-0.5 can still maintain 115 mAh/g, which is significantly better than that of Vac-0.5 (53 mAh/g). In addition, Nat-0.5 still maintains good cycle stability under high current. After cycling for 1000 cycles at 200 mA/g current density, the specific capacity almost has no attenuation, and can still be maintained at 242 mAh/g. The excellent electrochemical performance and simple and efficient preparation process make the Ti3C2Tx MXene film prepared by natural deposition method become an ideal flexible negative electrode for lithium-ion battery.
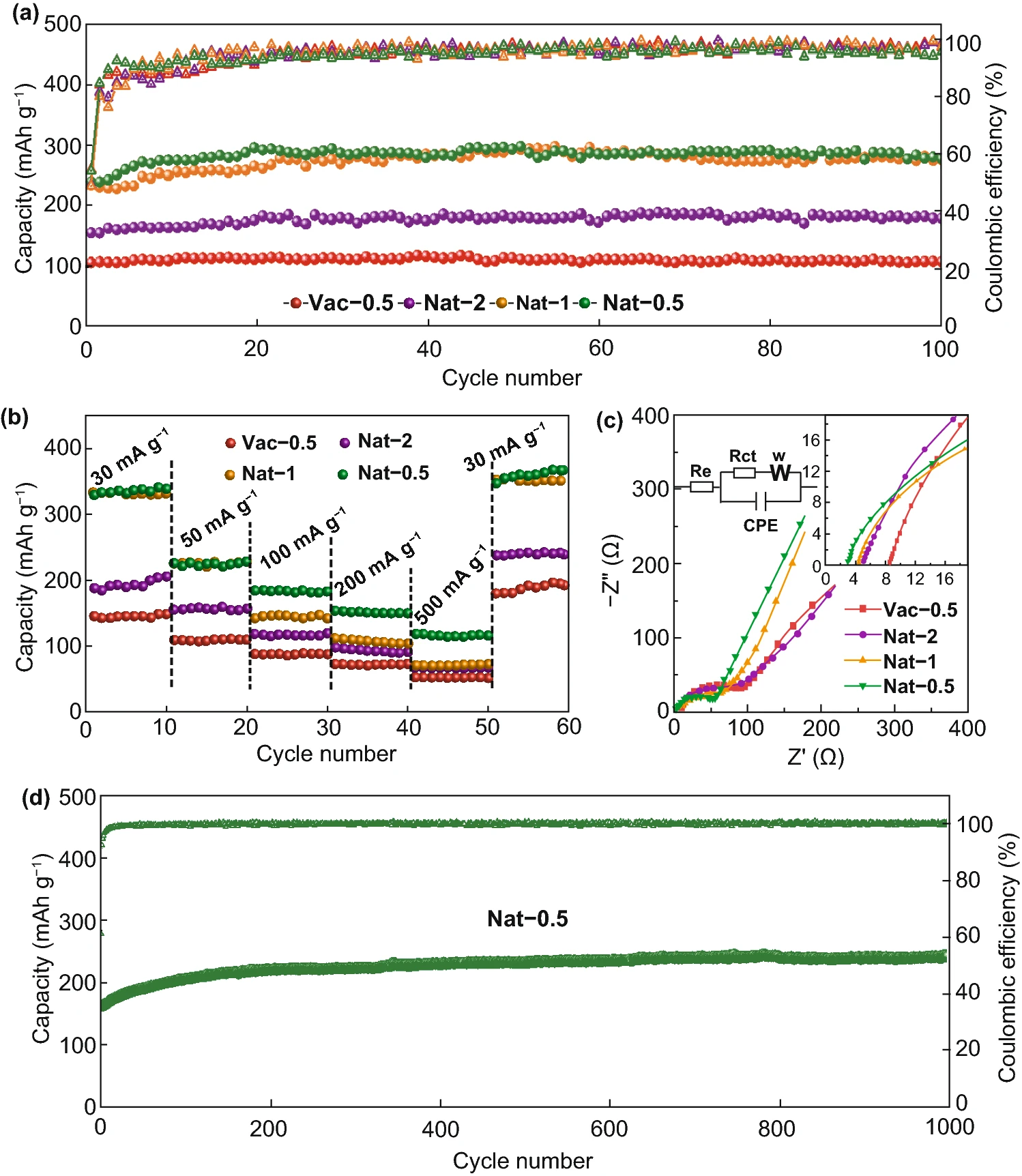
Figure 5.
The MXene membrane electrode prepared by vacuum filtration and natural sedimentation (a) cycle performance at 50 mA/g current density, (b) rate performance and (c) impedance spectrum, and (d) long cycle performance of Nat-0.5 at 200 mA/g current density.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.
18915694570
Previous: Wu Jin, Sun Yat-sen Un


