Weng Gengsheng of Ningbo University: dynamic coordinated control of multi-stimulus response of self-powered polymer hydrogels
[abstract].
The new self-powered hydrogel can change the electrical signal reversibly in response to the multiple stimuli of the surrounding environment, which is very attractive for the development of the next generation of intelligent sensing devices. Recently, the team of Associate Professor Weng Gengsheng of Ningbo University reported a newly designed self-powered metal hydrogel with rapid self-healing, reversible gel-sol transition and multi-stimulus response.
Hydrogels are crosslinked by dynamic metal-alanine (M-Ala) coordination. By assembling the hydrogel containing copper and zinc, the copper electrode is used as the cathode and the zinc electrode as the anode to produce a self-powered hydrogel that emits electrical signals in response to a variety of stimuli. External stimuli that can change the binding affinity of Ala ligands (e.g., heating, pH, and moisture) or exchange / replace Ala ligands (e.g., chelating agents) can be used to adjust the output electrical signal. The self-power design strategy combines multi-stimulus responsiveness, flexibility and self-power supply in a multi-stimulus response soft sensor. It also avoids the high temperature and energy-intensive process of integrating power sources in sensing devices. This work as a proof of concept paves the way for the manufacture of self-powered soft biomedical and wearable devices with multi-stimulus response. The related paper is published in ournal of Materials Chemistry A under the title Dynamic coordination of metal-alanine to control the multi-stimuli responsiveness of self-powered polymer hydrogels.
[guide to the main picture].
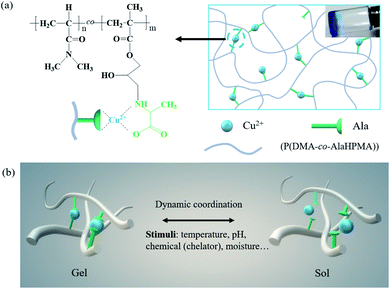
Figure 1.
(a) the scheme shows the coordination of Ala and Cu2+ ions as physical crosslinks in the P (DMA-co-AlaHPMA) network. (B) A picture showing the dissociation and recombination of Cu-Ala in response to multiple stimuli.
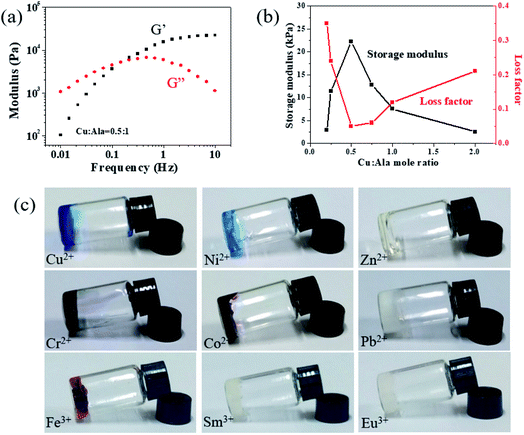
Figure 2.
(a) Rheological measurements show the relationship between storage modulus (G1) and loss modulus (G2) and frequency. (B) G1 and loss factor (tan δ) are plotted with Cu: Ala at a frequency of 10 Hz. (C) P (DMA-co-AlaHPMA) aqueous solution is gelated by the coordination of various metal ions with Ala.
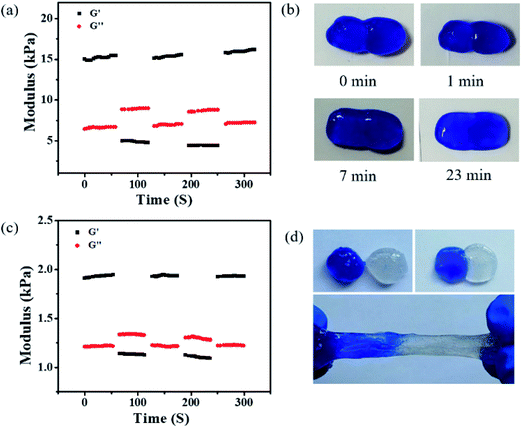
Figure 3.
(a) G1 and G2 come from continuous strain scanning of hydrogels containing Cu and alternately undergo small oscillatory strain (2%) and large oscillatory strain (500%) at an oscillation frequency of 1 Hz. (B) an image showing two copper-containing hydrogels healing within 23 minutes. (C) the rheological results under continuous strain scanning showed the changes of G1 and G2 of zinc-containing hydrogels. (d) describe the self-healing images of copper-containing hydrogels (blue) and zinc-containing hydrogels (colorless).
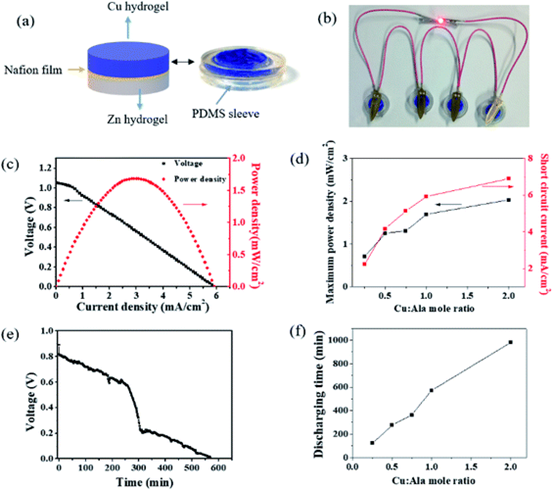
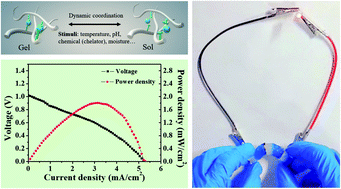
Figure 4.
(a) assemble a hydrogel containing copper and zinc with a Nafion film sandwiched between the two hydrogels. (B) A Cu-Zn primary cell was designed using Cu metal electrode as cathode and Zn as anode. (C) the relationship between voltage and power density and current density. (d) the maximum power density and the ratio of short-circuit current to Cu: Ala are plotted. (e) discharge the Cu-Zn primary battery with a discharge current of 1 mA cm-2. (F) the discharge time as a function of the Cu: Ala ratio.
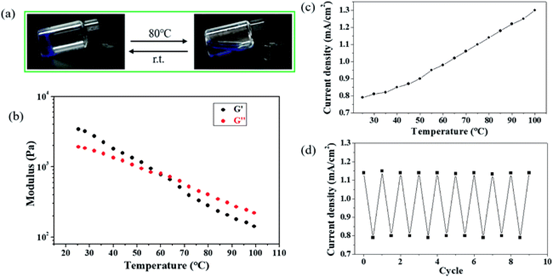
Figure 5.
(a) temperature response of Cu-Zn primary battery. (a) the image shows the reversible sol-gel transition of copper-containing hydrogels during heating / cooling switching. (B) G1 and G2 come from temperature-scanned copper-containing hydrogels with a frequency of 1 Hz. (C) the relationship between current density and temperature of Cu-Zn primary cell when heating copper-containing hydrogel. (d) the periodic change of current density during alternating heating (80 °C) / cooling cycles.
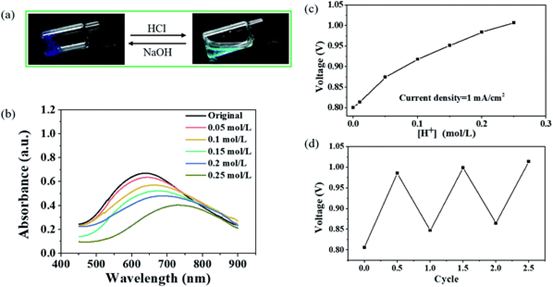
Figure 6.
(a) the image shows the reversible sol-gel transition and color change of copper-containing hydrogels after alternating addition of HCl/NaOH. (B) UV-vis spectra of copper-containing hydrogels at different H + concentrations. (C) the relationship between voltage and H + concentration drawn at a constant current density of 1 mA cm-2. (d) the periodic voltage change of Cu-Zn hydrogel cell with alternating titration of HCl/NaOH.
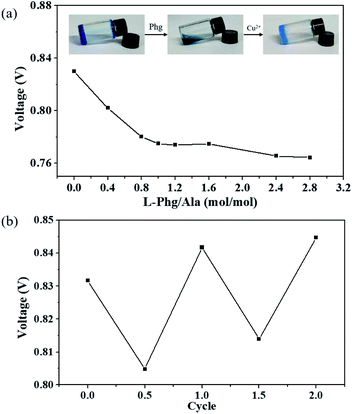
Figure 7.
(a) the effect of chelating agent 1-phenylglycinol (Phg) on the voltage of Cu-Zn primary cell. (B) the cyclic change of the voltage of Cu-Zn primary cell when Phg and Cu (NO3) 2 with the same molar amount are added alternately.
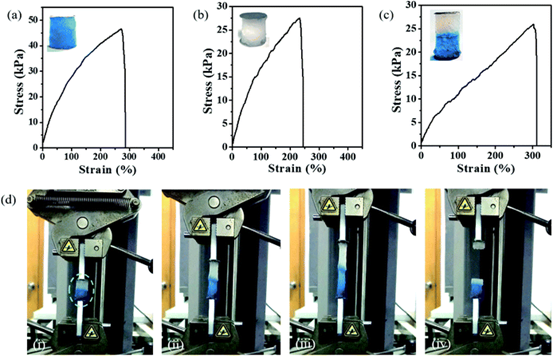
Figure 8.
Tensile test of P (DMA-co-AlaHPMA) / PDMA IPN hydrogel. (an and b) stress-strain curves of hydrogels containing Cu (a) and Zn (b) IPN. (C) the stress-strain curve of Cu-Zn primary battery. The photo images of the three test samples are given as illustrations in (amurc). (d) the image shows the stretching process of the Cu-Zn primary battery.
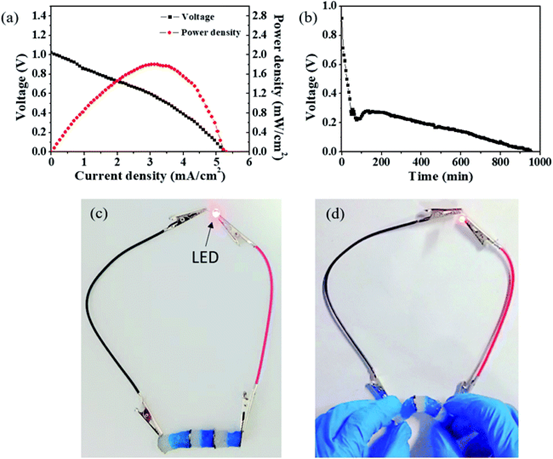
Figure 9.
(a) the relationship between voltage, power density and current density of Cu-Zn primary cell with IPN structure. (B) the Cu-Zn primary battery with IPN structure is discharged at a discharge current of 1 mA cm-2. (C) A LED is continuously lit by three Cu-Zn primary batteries connected in series. (d) the Cu-Zn primary battery connected in series can be used as a flexible and deformable battery.
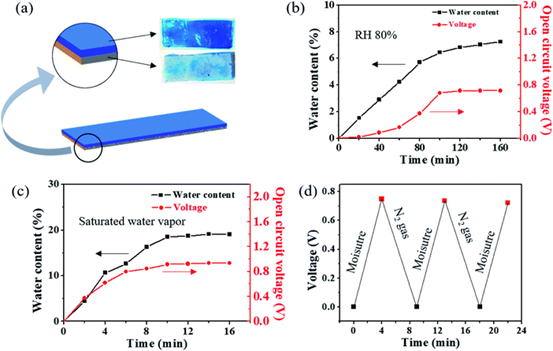
Figure 10.
(a) dehydrated IPN hydrogels containing copper and zinc are assembled into bilayer polymer sheets. When the relative humidity (RH) is 80% (b) and saturated water vapor (c), the water content and OCV of the Cu-Zn primary cell are plotted over time during the water absorption process of the polymer sheet. (d) the reversible change of OCV of Cu-Zn primary cell when saturated water vapor and dry N2 gas are applied alternately.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.
18915694570
Previous: Ti3C2Tx MXene nano-she


