Bioglass scaffold material for bone tumor therapy: 3D printing of ultra-thin two-dimensional niobium carbide MXene nanoscale
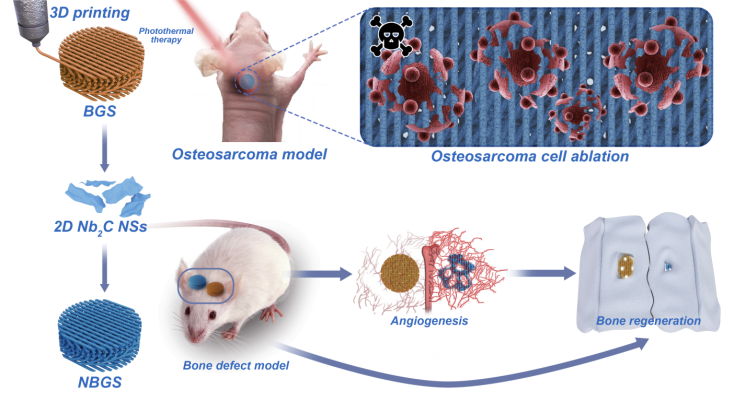
Postoperative recurrence of bone tumor and secondary bone defect after tumor resection are thorny problems faced by clinicians. At the same time, the multi-functional biotherapy materials with the ability of tumor ablation and inducing bone regeneration (with both treatment and repair function) are expected to be developed for clinical bone tumor treatment. Two-dimensional niobium carbide (Nb carbide C) MXene has good biocompatibility and biodegradability, and has inherent light response and photothermal conversion properties in the second near infrared (NIR-II) biological window. In this work, 2D Nb C MXenes was integrated into 3D porous bioglass scaffold to achieve the multiple functions of killing bone tumor cells and driving new bone formation by vascularization, which provides a new type of biotherapy scaffold material for bone tumor treatment.
The highlight of this article.
1. 2D niobium carbide MXene nanosheets and 3D printed bioglass scaffolds were used to prepare biocomposite scaffolds with the functions of photothermal therapy and bone repair.
two。. The biological composite scaffold material can achieve efficient photothermal ablation of osteosarcoma under the NIR-II biological window.
3. The biological composite scaffold material has good bone conductivity and bone induction, and can drive the vascularization of the defect site and promote bone regeneration.
Content introduction.
Early surgical resection and chemotherapy are common methods for the treatment of bone tumors, but it is still challenging to prevent recurrence and fill bone defects caused by resection. In this paper, Gao Junjie, Zhang Changqing, the sixth peoples Hospital affiliated to Shanghai Jiaotong University, Chen Yu, Shanghai University, reported a method of combining two-dimensional ultra-thin niobium carbide MXene nanosheets excited by near-infrared light into 3D printed bioglass scaffolds (NBGS) for the treatment of osteosarcoma. The integrated 2D Nb C-MXene has light response and high tissue penetration depth in the second near infrared (NIR-II) biological window, which makes it effective in killing bone cancer cells. In addition, the material can significantly promote angiogenesis. The calcium and phosphate released during the degradation of the scaffold can promote the mineralization of new bone tissue. Niobium carbide MXene composite stent has many functions, such as killing bone tumor cells, promoting angiogenesis and bone regeneration. Niobium carbide composite stent is a kind of implantable biotherapy material with good curative effect on bone tumor.
Guided reading of picture and text.
Synthesis and characterization of I 2D Nb Crystal C MXene NSs.
2D Nb powder C MXene NSs was prepared by chemical stripping method. SEM images show that the Nb-bonded AlC ceramics (MAX phase) prepared by solid-state sintering have dense lamellar structure. As shown in figure 1, the SEM image and the corresponding element content show that the MAX phase ceramics are Nb, Al and C ternary compounds (figure 1b). After treatment with HF, a multi-layer structure (MXene) was formed, and the content of Al decreased significantly (Fig. 1D). After further insertion of TPAOH, a few layers of Nb roomC NSs were obtained. Compared with Nb AlC, the signal intensity of Nb element in Nb C NSs is significantly enhanced, while the signal intensity of Al element is decreased. Consistent with XPS data, Raman spectra show that the loss of NSs of element Al in Nb powder C is more significant than that in Nb powder AlC powder. After HF and TPAOH treatment, the Nb texture CNSs showed a layered structure.
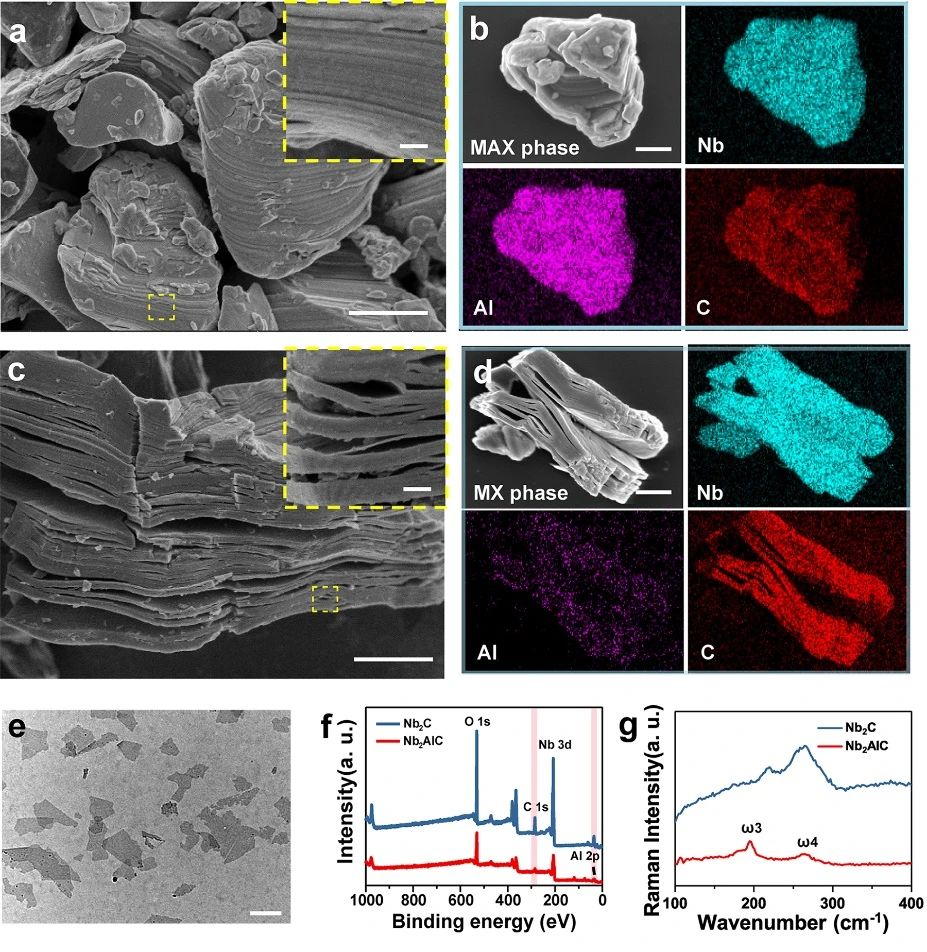
Figure 1.
Preparation and characterization of ultra-thin 2D Nb powder C MXene NSs. (a) SEM images of the corresponding elements (Nb, Al and C) of Nb AlC ceramics; (c) SEM images of multilayer Nb CMXene and its corresponding elements (Nb, Al and C); (e) TEM images of ultra-thin Nb CMXene NSs; (f) X-ray photoelectron spectroscopy (XPS) of Nb AlC and Nb CNSs; and (g) Raman spectra of Nb Alc and Nb CNSs.
Synthesis and characterization of II 3D NBGS.
Intuitively, from BGS, 0.25 NBGS, 0.5 NBGS to 1.0 NBGS, the color of the scaffold gradually changed from white to black, but the 3D clear micropore structure did not change during the modification of Nb / C NSs. In addition, with the increase of the concentration of Nb C NSs, the number of Nb C NSs on the surface of NBGS increased, while the micropores decreased. The cross-sectional morphology of NBGS and magnified interface SEM images show the successful integration of Nb C NSs and BGS.
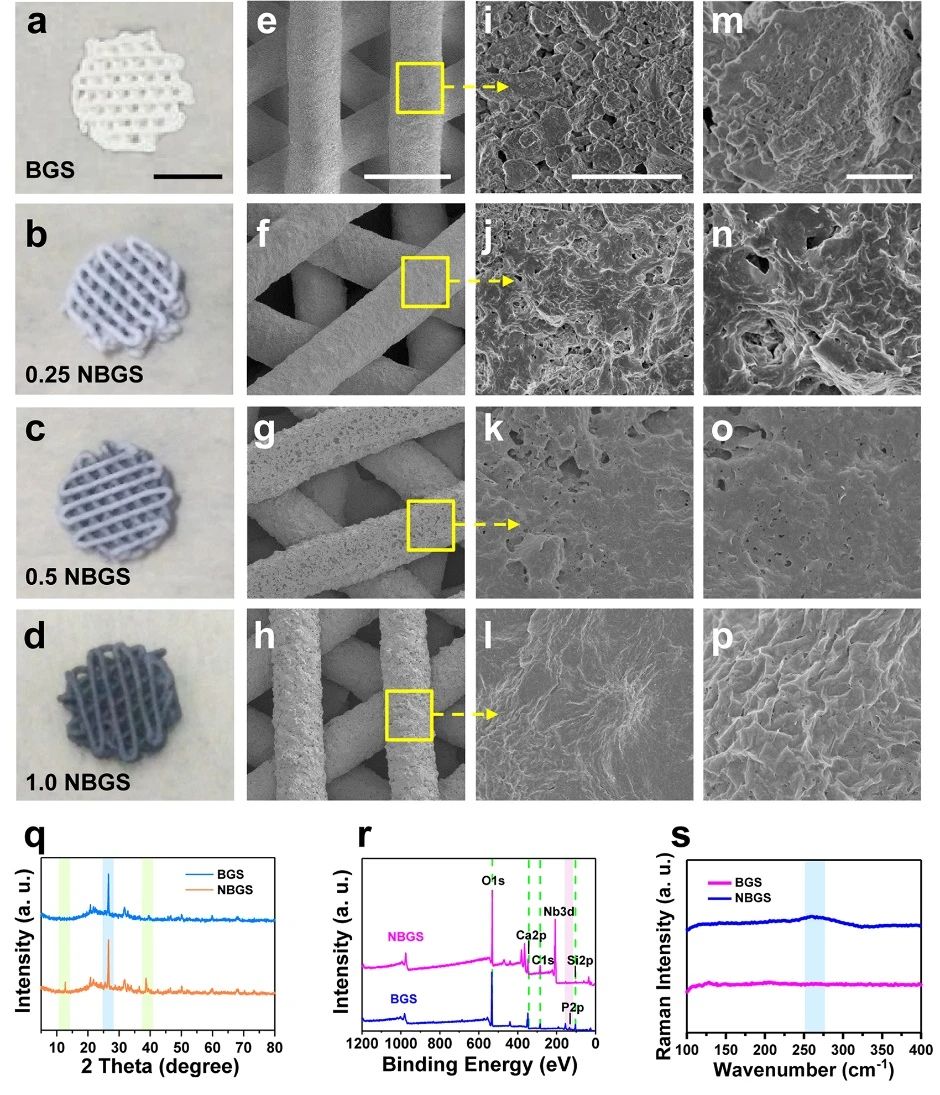
Figure 2.
Synthesis and characterization of 3D NBGS. (Amurd) digital photos of BGS and NBGS with three-dimensional geometry; (eMurp) SEM images with different magnification; (Q) XRD spectra of BGS and NBGS; (r) XPS spectra of BGS and NBGS; and (s) Raman spectra of BGS and NBGS.
Photothermal properties and anti-tumor properties of III NBGS good results were obtained when NBGS was used in anti-tumor experiments in vivo. NIR-II (1064 nm laser) thermal ablation was performed at the tumor site 24 hours after stent implantation. Within 3 min of NIR irradiation, the surface temperature of xenografts increased to ~ 56 ℃, which was significantly higher than that of the control group (~ 40 ℃). The next day, the randomly collected tumor samples were stained with Hype, TUNEL and Ki-67, and the ablation effect of osteosarcoma was observed. TUNEL and hue staining showed that there were few apoptotic tumor cells in BGS group, BGS+NIR group and NBGS group, but obvious nuclear dissociation and a large number of tumor cells died in NBGS+NIR group. Ki-67 staining also supported the results of TUNEL and hue staining. These results show that NBGS can effectively kill tumor cells. In order to evaluate the acute toxicity of NBGS, the main organs of mice were taken 24 hours after laser irradiation. There was no obvious inflammatory infiltration or other pathological abnormalities in both the treatment group and the control group, indicating that NBGS has good biological safety for photothermal bone tumor ablation.
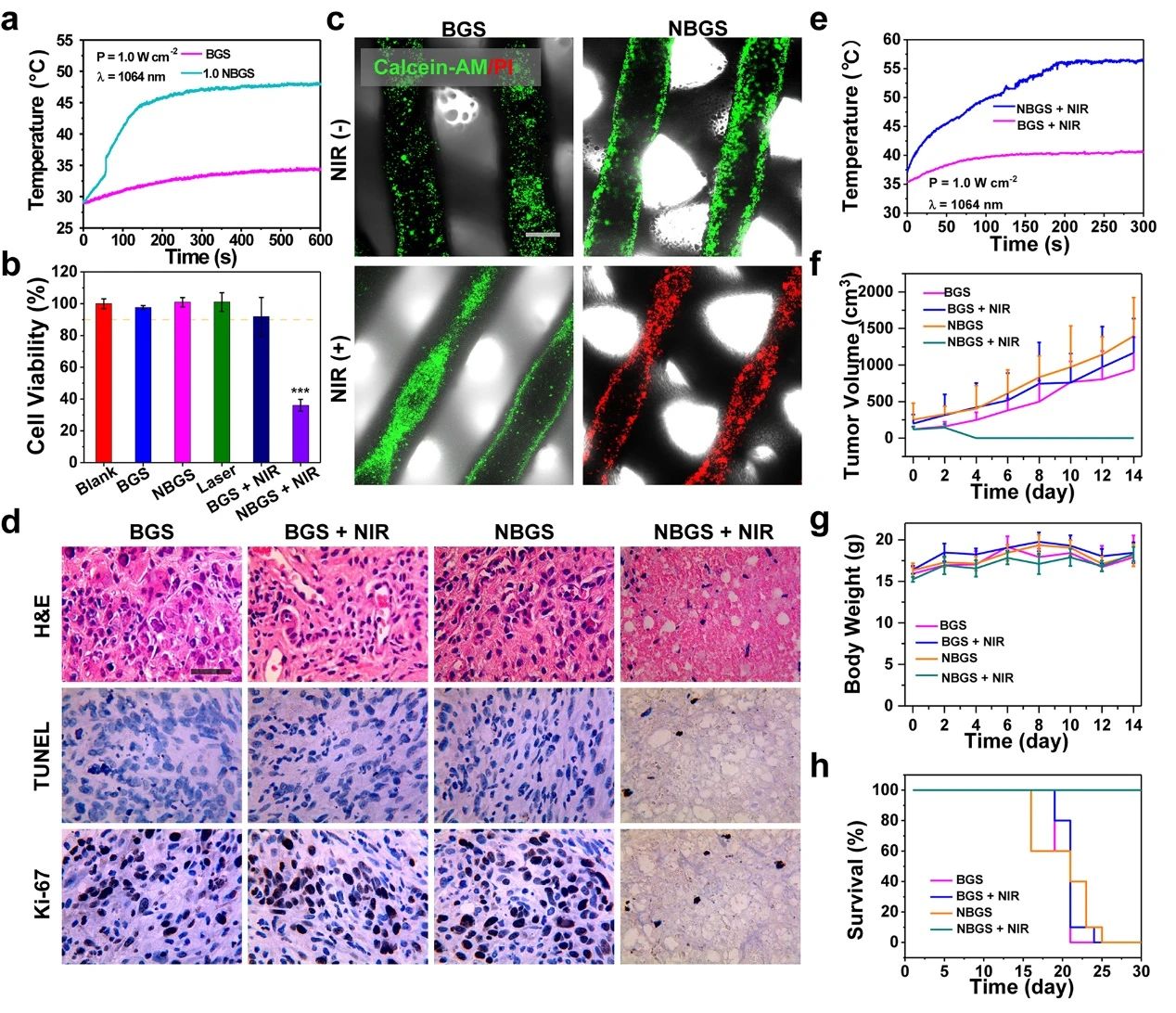
Figure 3.
Photothermal properties and anti-tumor properties of BGS/NBGS. (a) the temperature change curve of 1.0NBGS and BGS in PBS; (b) the relative activity of Saos-2 cells after different treatments; (c) Calcein-AM/PI staining of different treatment groups; (d) the Hype, TUNEL and Ki-67 staining images of tumor tissue; (e) the temperature curve of mice implanted with 1.0NBGS and BGS after NIR-II laser irradiation; (f) the tumor volume of tumor-bearing mice with different treatment regimens. (G) body weight of tumor-bearing mice, (h) survival curve of mice in different treatment groups.
IV NBGS can stimulate angiogenesis in vivo and in vitro.
Blood vessels play an important role in the whole process of bone repair. Angiogenesis is closely related to bone formation. When a bone defect occurs, osteogenic progenitor cells are recruited to the injured site through new blood to participate in osteogenesis. This work analyzed the difference between BGS and NBGS in promoting angiogenesis in vitro and in vivo (see figure 4). The results of scratch test and migration experiment showed that NBGS significantly improved the migration ability of HUEVCs. In the tube forming experiment, the number of branches in the NBGS group was more than that in the control group. In order to clarify the mechanism of NBGS promoting angiogenesis, we used QPCR method to detect the expression of tubule-related genes. Compared with BGS group, NBGS can continuously promote the expression of VEGF-B and FGF2, indicating that NBGS has a better function of promoting angiogenesis. In the in vivo experiment, NBGS was implanted into the defect of rat skull for 3 weeks, and the angiogenesis around the stent was observed by Microfil perfusion. The experiment shows that there is a denser vascular network around NBGS. The results in vivo were consistent with those in vitro. The results show that Nb released from NBGS can stimulate vascularization and is an ideal element to promote osteogenic coupling.
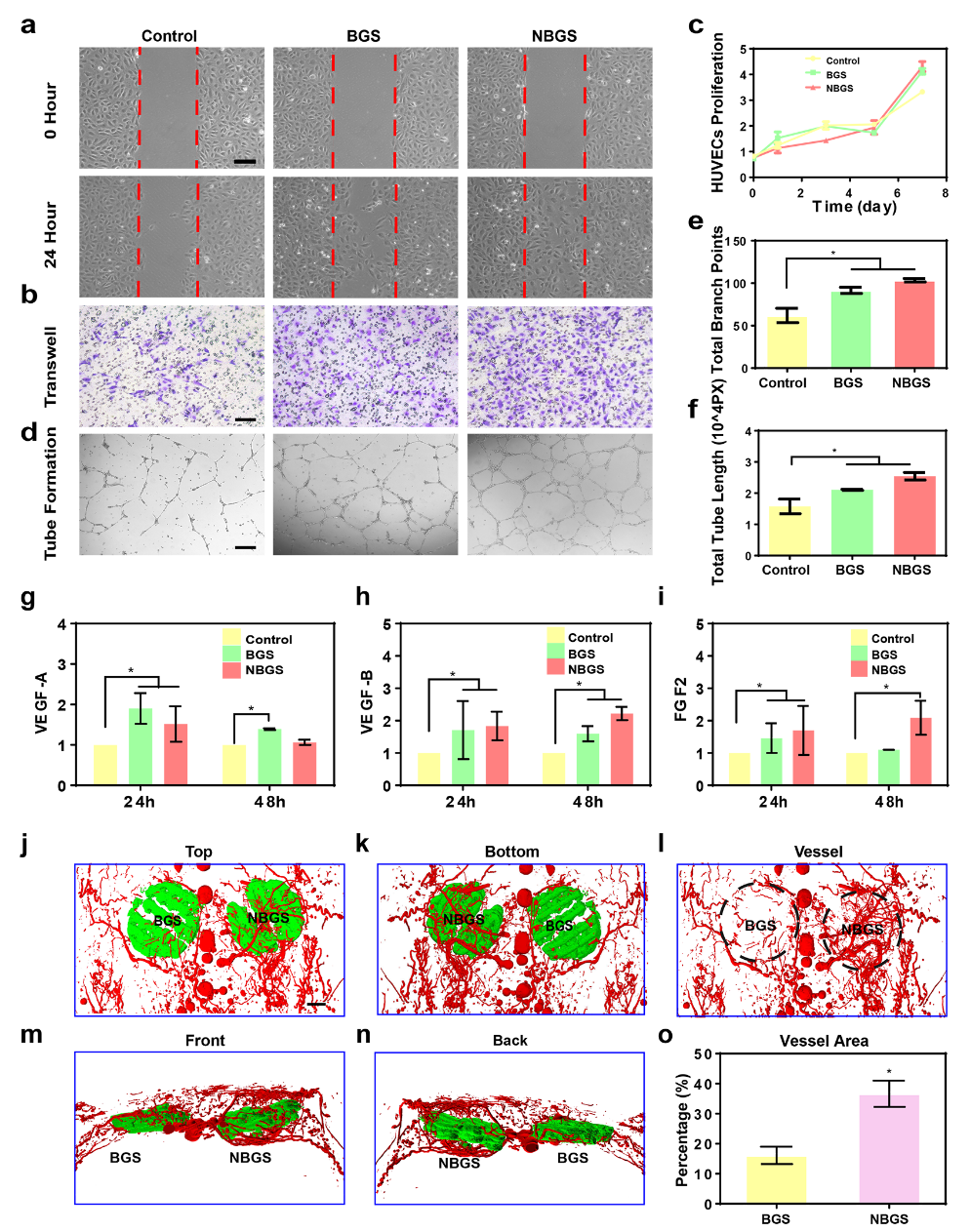
Figure 4.
BGS and NBGS stimulate angiogenesis in vivo and in vitro. (a) scratch test; (b) migration test; (c) proliferation test; (dmurf) tube formation effect analysis; (jmurn) three-dimensional reconstruction image of stent implantation in vivo; (o) quantitative analysis of neovascularization.
Evaluation of osteogenic effect of V-NBGS in vitro and in vivo.
Hydroxyapatite plays an important role in the proliferation and osteogenic differentiation of mesenchymal stem cells (MSC). The closer the Ca/P ratio is to the composition of hydroxyapatite, the stronger the mineralization ability is. The Ca/P ratio of NBGS is beneficial to the osteogenic differentiation and new bone mineralization of MSC, and has a better ability to promote bone regeneration in vivo. The proliferation of hBMSCs co-cultured with NBGS was better. In addition, the expression of osteogenesis-related genes COL1, OCN and OPN was significantly up-regulated. The result of alizarin red staining was consistent with that of QPCR. Therefore, NBGS has good bioactivity, biocompatibility and osteogenic properties, and can be used as a good biomaterial to drive bone regeneration. SD rat bone defect model is one of the most commonly used models to study bone defect regeneration. The cranial bone defect model of SD rats was established, and the effect of NBGS on promoting bone regeneration was evaluated by micro-CT after NBGS and BGS were implanted. The results showed that the ability of NBGS to promote bone regeneration was significantly higher than that of BGS group.
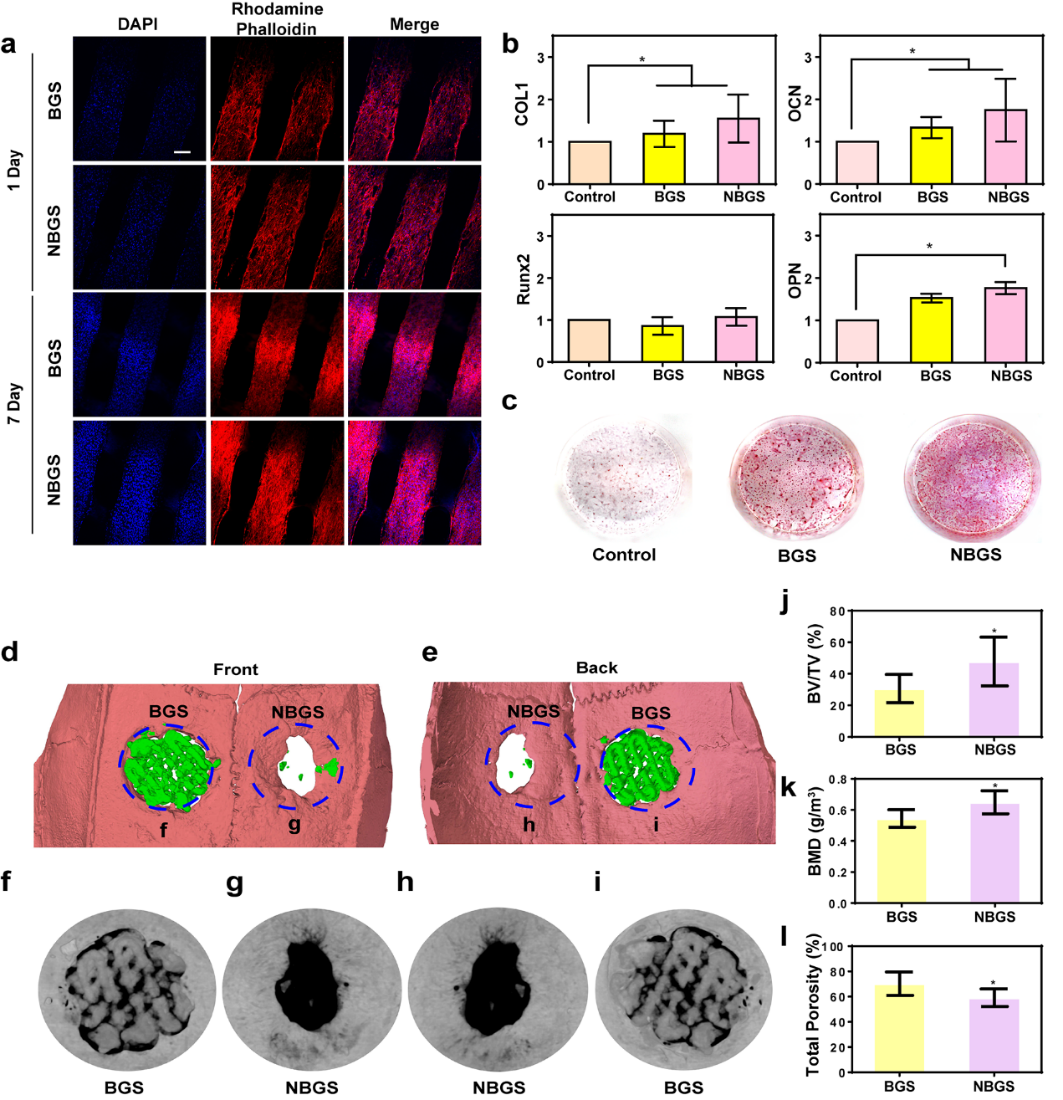
Figure 5.
The effect of BGS/NBGS on osteogenesis. (a) confocal images of hBMSCs co-cultured with BGS/NBGS; (b) expression of hBMSCs osteogenic genes (COL1, RUNX2, OCN, OPN) in control group, BGS group and NBGS group; (c) alizarin red staining; (dMul) micro-CT to evaluate bone regeneration in each group.
The repair of rat skull was further evaluated by fluorescent trichrome test (figure 6). Compared with BGS group, NBGS scaffold showed better performance of inducing bone regeneration. The results of Hype staining and Masson staining showed that there were a large number of mineralized bone tissue in the bone defect implanted with NBGS, and there was no obvious residual scaffold. In addition, Goldner staining showed that there was a new osteoid (red tissue) mixture around the residual material in the defect area of the BGS group, while the bone tissue around NBGS had been heavily mineralized (green tissue). The results show that the scaffold has good ability of bone regeneration and degradation.
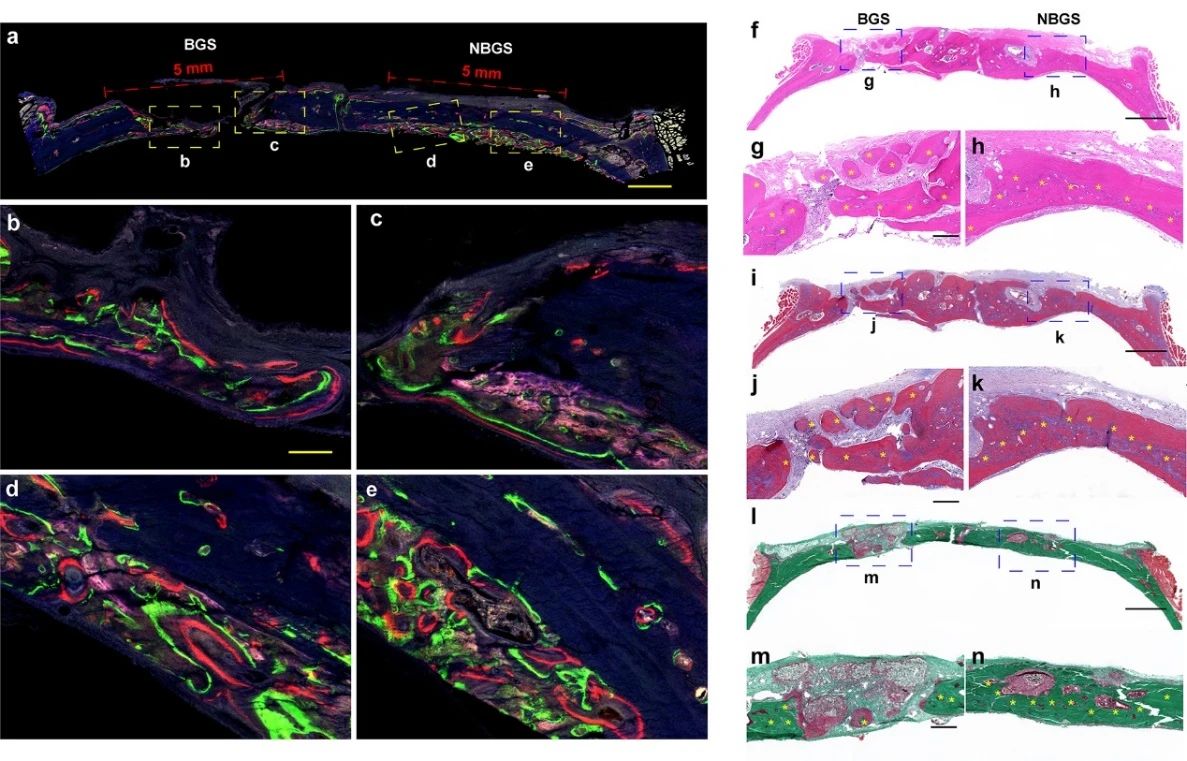
Figure 6.
Fluorescence imaging and histological staining of newborn bone. At the 2nd, 4th and 6th week, tetracycline hydrochloride (blue fluorescence), Calcein AM (green fluorescence) and alizarin red (red fluorescence) were injected subcutaneously, and the different colors of fluorescence represented the new bone tissue at different time, (Fmurk) Hendry staining, Masson staining and Goldner staining.
In animal experiments, it was found that the new bone grew along the pores of the stent (as shown in figure 7). These results show that bone regeneration is good and bone conductivity is good under the guidance of materials in vivo. Figure 7g-i shows the formation of new bone tissue in different periods. At the 8th week, a large number of fibroblasts were distributed in the space of the stent. At the 16th week, the biodegradation of the scaffold was accompanied by the gradual filling of red osteoid, which showed the coordination of bone formation and scaffold degradation in the NBGS group. The previous study confirmed that Nb C can significantly promote angiogenesis, through rich blood supply can gather more immune cells around the defective site, and accelerate the degradation of NBGS. In addition, a large number of nutrients, oxygen and bone marrow mesenchymal stem cells are transported to the bone defect through neovascularization to promote bone regeneration. The degradation of NBGS provides enough space for bone remodeling. The calcium and phosphate released during the degradation of the scaffold can promote the mineralization of new bone tissue. In contrast, the control group due to the scarcity of neovascularization and insufficient supply of raw materials significantly slowed down the degradation rate of BGS stents.
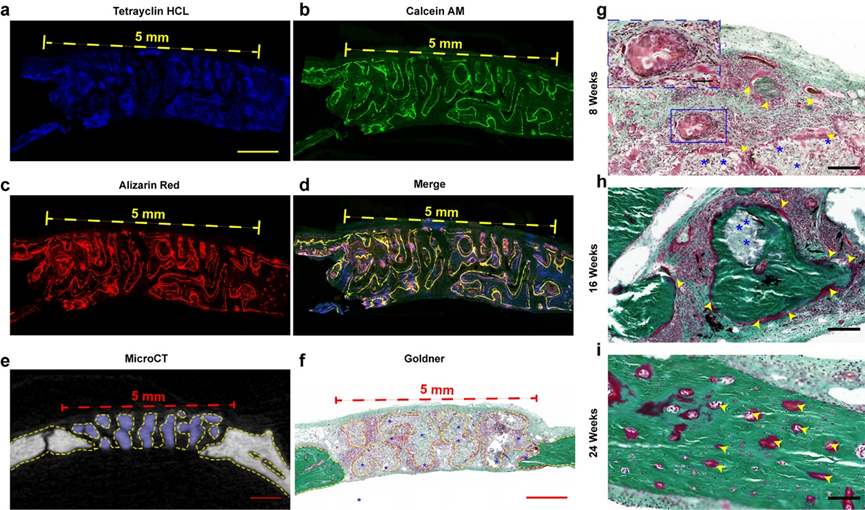
Figure 7.
Material-guided bone tissue regeneration in vivo. Confocal imaging of new bone in defect area, (e) microCT cross-sectional imaging, (f) Goldner staining in NBGS group at 8th week, and Goldner staining in NBGS group at 8th, 16th and 24th week.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.
18915694570
Previous: AFM Review of Harvard


