Wang Qigang, Tongji University, Angew: super fast gel and tissue repair liquid for tissue repair.
abstract
In situ gelation of injectable precursors is desirable in the field of tissue regeneration, especially when irregular defects are filled. At present, the driving force of rapid gelation includes the phase transition of thermosensitive copolymer, the click chemistry reaction with tissue composition and the metal coordination effect. However, the rapid formation of tough hydrogels is still a challenge. Inspired by aerobic metabolism, Professor Wang Qigang of Tongji University proposed a cascade enzyme polymerization triggered by glucose oxidase and ferrous glycine catalyzed by tissue liquid, which was used for super fast gelation of acrylonylated chondroitin sulfate and acrylamide.
The efficient production of carbon free radicals and macromolecules helps to accelerate the polymerization automatically, which can be used to increase the soft tissue in bone defects. Copolymer hydrogel demonstrated the ability of cartilage regeneration. As the first example of in situ polymerization using artificial enzyme complexes, this work provides a bionic method for designing intensity adjustable hydrogels for biological implants and biological printing applications. The related papers were published in "Angewandte Chemie International Edition" under the title of tissue fluid triggered enzyme polymerization for ultrafast gelation and cartilage repair.
Main picture
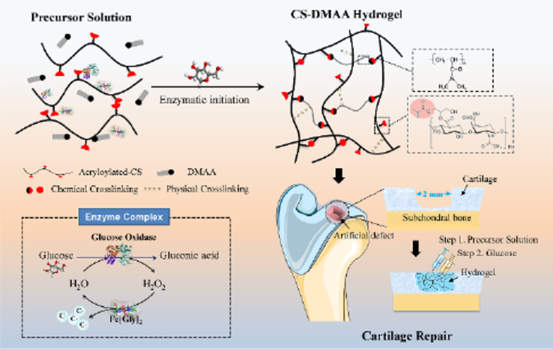
Schematic diagram 1 CS-DMAA hydrogel preparation. The molecular structures of acrylated CS and poly (DMAA) were given. The hydrogels are used as tissue filling agents through in situ injection and glucose responsive hydrogels.
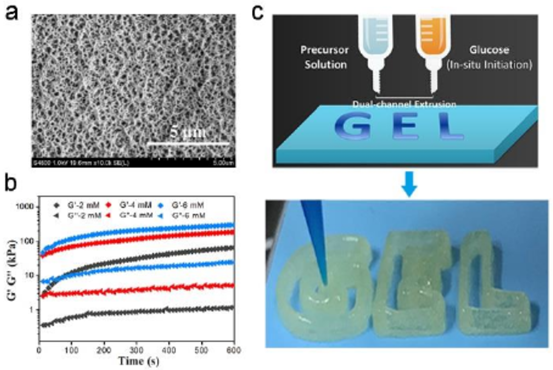
Fig. 1 SEM image of (a) CS-DMAA hydrogel. B) the time curve of CS-DMAA hydrogel during rheological test. c) Schematic and photographic images of a dual channel 3D printing process using precursor solution and glucose solution as ink.
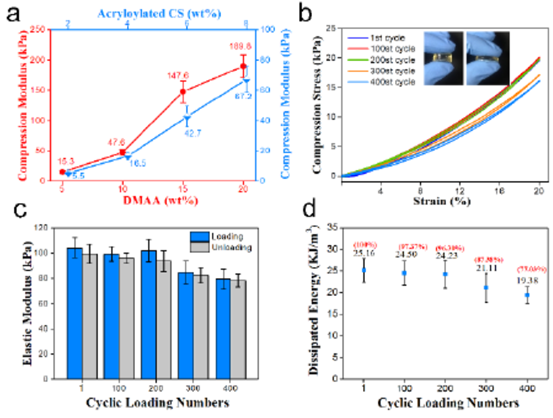
Fig. 2 (a) compression modulus of CS-DMAA hydrogel containing different concentrations of Acryloylated-CS or DMAA. B) the cyclic compression curve of CS-DMAA hydrogel deformed to 20%. C) the change of elastic modulus of CS-DMAA hydrogel during cyclic compression. The dissipative energy of D) CS-DMAA hydrogel during cyclic compression.
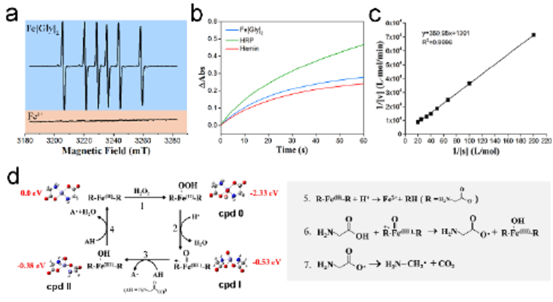
Fig. 3 (a) EPR spectra of carbon radicals formed by GOx / Fe [Gly] 2 and GOx / Fe2 + in the presence of glucose (b) enzyme kinetic curves of Fe [Gly] 2, HRP and hemin under the same reaction conditions( c) Lineweaver Burk diagram of Fe [Gly] 2 as HRP simulant( d) The proposed catalytic mechanism of Fe [Gly] 2 and the production mechanism of carbon free radicals.
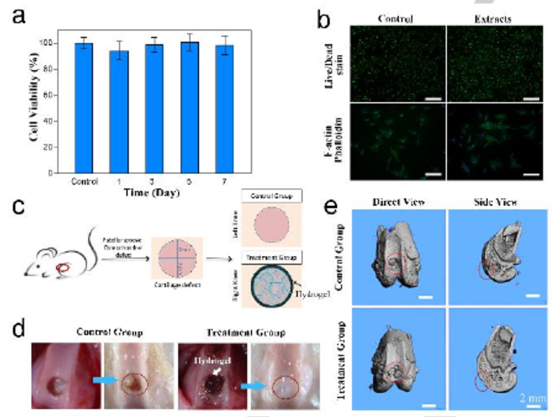
Fig. 4 (a) the viability of chondrocytes cultured in hydrogel extracts was more than 7 days. B) fluorescence images of live / dead and F- actin phallin stained chondrocytes cultured in Dulbecco s Modified Eagle medium and hydrogel extracts. C) a schematic diagram of animal experiments using hydrogels to repair cartilage. d) The patella photos of the control group and the treatment group before and after treatment( e) The micro CT images of the patella in the control group and the treatment group were compared.
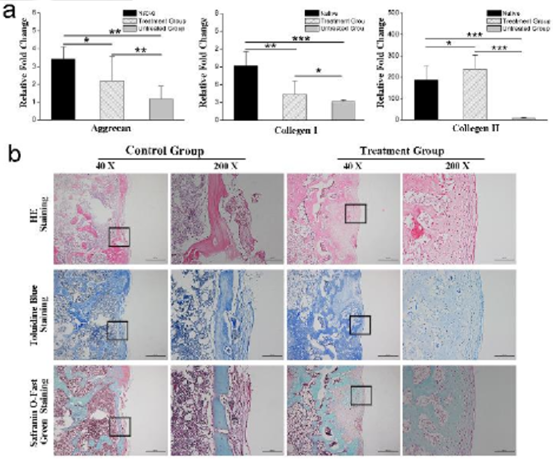
Fig. 5 (a) gene expression of proteoglycan, collagen I and collagen II( b) He staining, toluidine blue staining and safranine O staining were used to observe the histological sections.
This information is from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately
18915694570
Previous: Natural chemistry : ac


