Advanced Science: 3D bioprinting based on entangled micro-beam hydrogel
Extrusion-based 3D bioprinting technology has been widely used to create a variety of functional tissue-like structures, including cardiac muscle, skeletal muscle, liver, skin, bone, and cartilage. Generally, bio-inks are prepared from hydrogel precursors. By changing the rheological properties of the hydrogel precursor, the bio-ink can be deposited on the support platform as a continuous gel filament during extrusion. Compared with liquid bio-ink, microgel bio-ink is considered to be a new type of bio-ink, which has inherent shear thinning and shear recovery properties. At the same time, due to the inherent voids between the microgels, compared with bulk gel materials, the cells in the microgel bio-ink have higher viability, diffusion and migration capabilities.
Recently, the team of Professor Marcy Zenobi-Wong from the Department of Health Science and Technology of the University of Zurich squeezed the massive hydrogel through a grid of micrometer-sized pores to decompose the hydrogel into a micro-beam gel network. The gel scaffold formed by co-deposition of chondrocytes and gel microbeams in 3D bioprinting can induce chondrocytes to form a rich extracellular matrix, and its compressive modulus is significantly improved. The construction strategy of micro-beam hydrogels in this research provides a new preparation method for 3D printing bio-inks. Related research paper: "3D Bioprinting of Macroporous Materials Based on Entangled Hydrogel Microstrands" was published on Advanced Science.
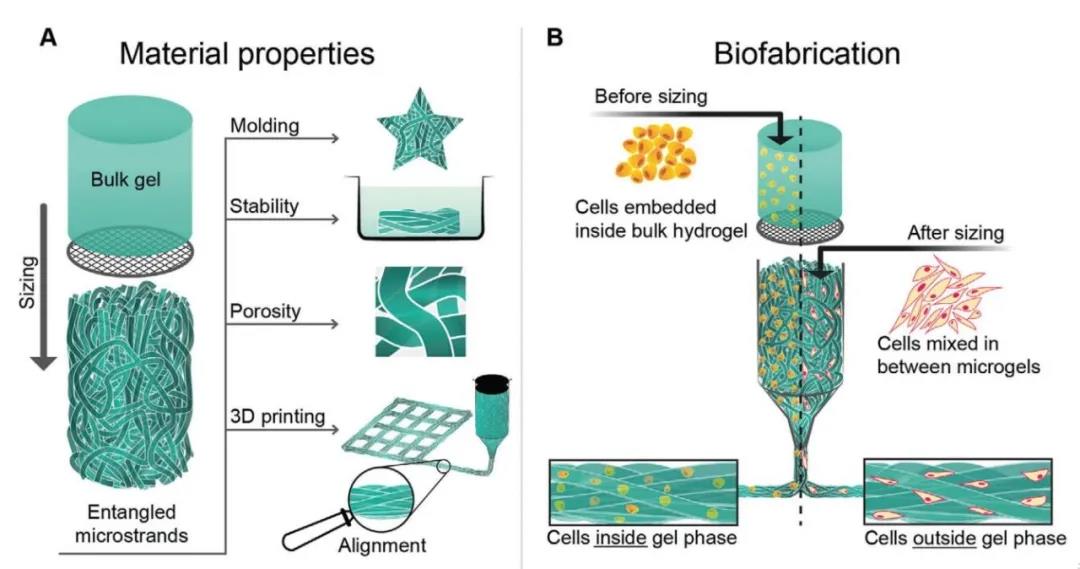
Figure 1 Schematic diagram of the construction and 3D bioprinting of entangled micro-bundle gel network
Gel material:
Methacryloyl hyaluronic acid (HA-MA), methacryloyl gelatin (GelMA), gelatin
Process:
UV cross-linked HA-MA (concentration 2% w/v, grafting rate 28%) or GelMA (concentration 2% w/v, grafting rate 92%)/gelatin block hydrogel through holes of 40~100μm The sieve is mechanically squeezed. This process decomposes the gel mass into gel micro-beams, which are randomly entangled with each other to form a structured material composed of a high aspect ratio hydrogel.
1. Stability and macro porosity of entangled micro-bundle gel:
In order to study the tunability of 3D printing entangled microbeam gels, the researchers prepared a series of HA-MA system gels with different crosslinking strengths by adjusting the UV crosslinking time (20s, 60s, 180s). In contrast, the granular hydrogel was immersed in PBS at 37°C for 7 days. The bulk microgel collapsed within 1 hour, while the entangled micro-bundle hydrogel had a long-term cohesion in an aqueous medium. In order to evaluate the porosity of the HA-MA entangled microbeam gel, the researchers immersed the gel in a high molecular weight dextran fluorescent dye. Due to the large molecular weight of dextran, Figure 2H shows that the fluorescently labeled dextran can only enter between the gel bundles and cannot penetrate the gel phase of the hydrogel. Based on this, researchers can use it to calculate the gel The porosity (Figure 2).
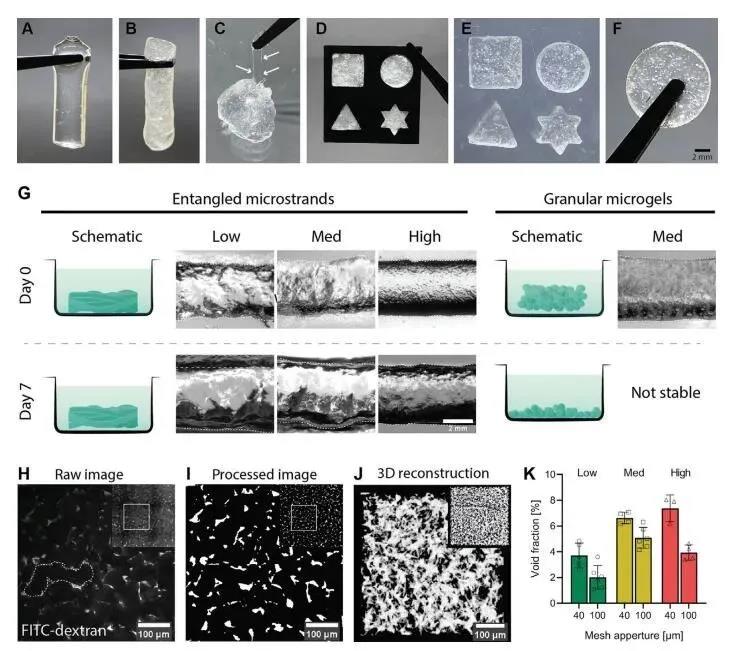
Figure 2 Stability and macro porosity of entangled micro-bundle gel network
2. Shear thinning and shear recovery characteristics of HA-MA entangled micro-bundle gel:
The entangled micro-beam type gel exhibits shear thinning rheological properties, which is necessary for extrusion-type 3D bioprinting. Through rheological measurement, the shear recovery rate of HA-MA microbeam gel with different crosslinking strength is different, and it changes with the difference of the sieve aperture. Through comparison, the researcher pointed out that the entanglement of medium crosslinking strength is different. The shear recovery rate of the microbeam type gel sample after initial shearing is 81.8% (mesh mesh size 40 μm) and 82% (mesh mesh size 100 μm), which is significantly better than the other two groups and can be used as an excellent 3D Print bio-ink (Figure 3).
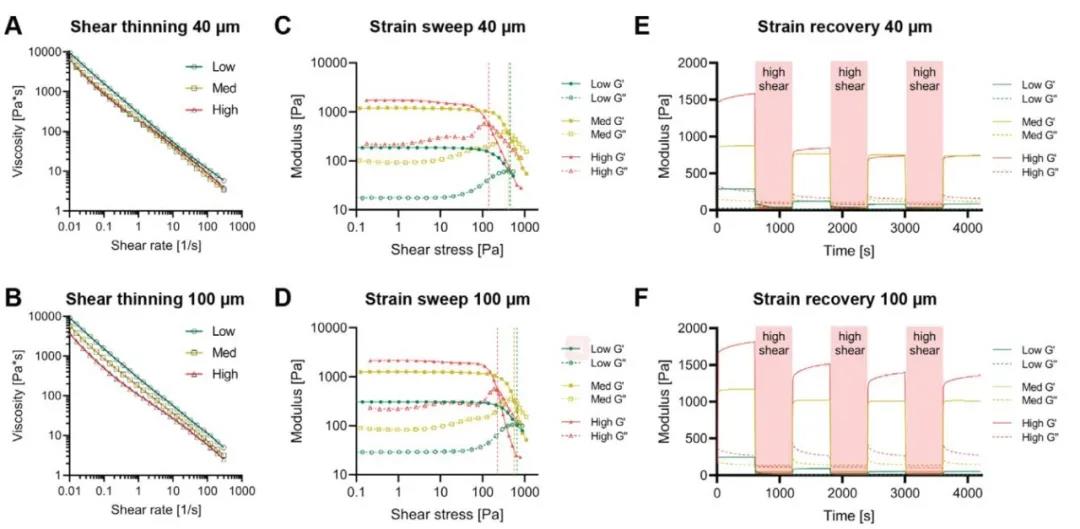
Figure 3 Rheological properties of HA-MA entangled micro-beam gel network
3. 3D printing characteristics of entangled micro-bundle gel:
Based on the HA-MA microbeam gel, the researchers 3D bioprinted a two-layer grid structure. The higher the cross-linking rate, the more easily the gel sample breaks under a shorter stretching distance, and the higher the printing accuracy. In addition, considering the rheological properties of the entangled micro-beam type hydrogel, the entangled micro-beam type gel-like sample with medium cross-linking strength is the most excellent bio-ink. The rheological properties and printing performance of the two pore sizes (40 μm and 100 μm) are not significantly different, but the pore size has a significant effect on the porosity, so this parameter can be selected according to the actual application to adjust the macro porosity of the gel. In addition, the researchers also tested other hydrogel systems (GelMA, carrageenan, enzyme or chemically cross-linked HA, etc.). After pre-cross-linking and filtering to form a micro-beam gel, it was then subjected to extrusion-type 3D bioprinting. The test results show that all the gel systems can form entangled microfilaments and 3D print out a grid scaffold.
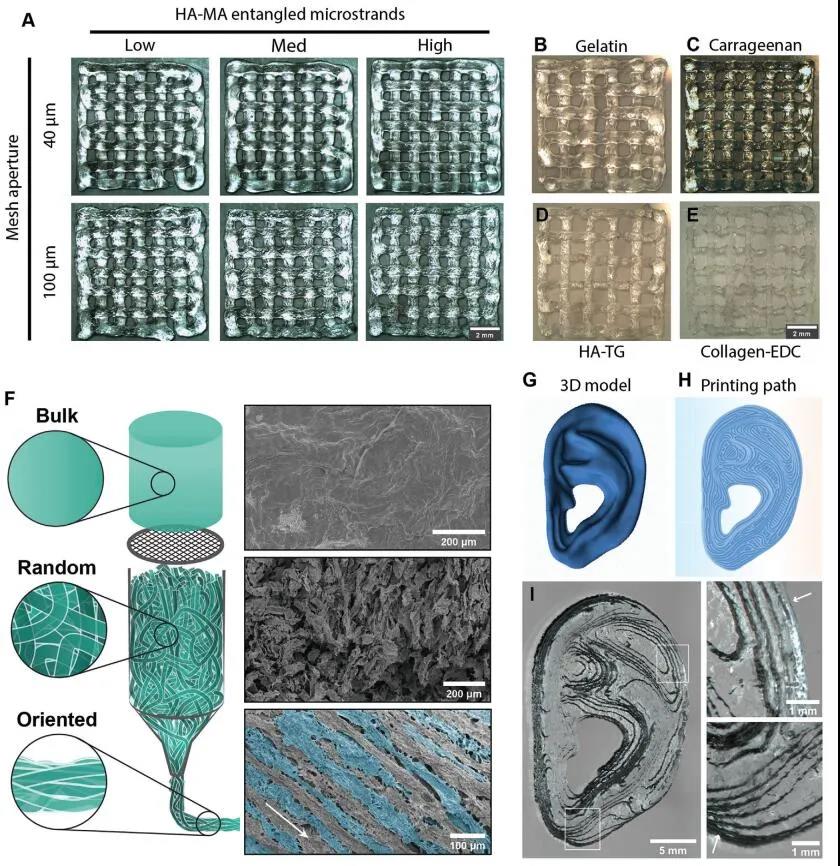
Figure 4 Extrusion 3D printability and anisotropy of entangled micro-bundle gel
4. 3D bioprinting of tangled micro-bundle gels loaded with cells:
The researchers used two methods to explore the cell-loading capacity of the entangled microbeam gel: Method one, first, the cells are embedded in the bulk hydrogel, and then the hydrogel is squeezed into gel microbeams; Method Second, the extruded gel microbeams are mixed with the cell suspension, so that the cells occupy the pores between the microbeams. Using method 1, the researchers chose gelatin combined with GelMA hydrogel to explore the ability of the tangled micro-bundle gel to support mouse myoblasts (C2C12). Calcein AM staining combined with Hoechst staining showed that the cells were fused and formed along the direction of microfilaments. Arrangement of multinucleated myotubes (Figure 5). Using method two, the researchers selected HA-MA hydrogel microbeams to compound with chondrocytes. The results showed that chondrocytes showed a circular phenotype after 3D bioprinting, but proliferated and displayed longer after 7 days and 21 days of culture. Shuttle type (Figure 6).
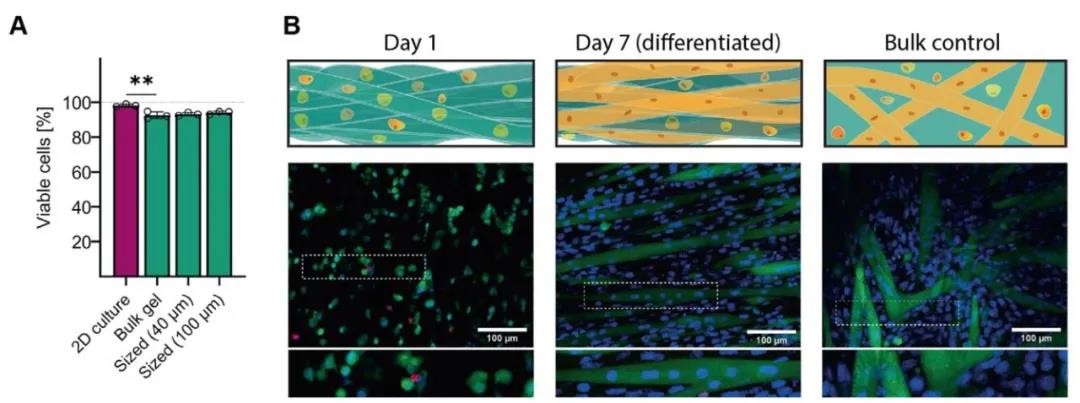
Figure 5 Tangled microbundle gel can induce myoblast formation in myoblasts
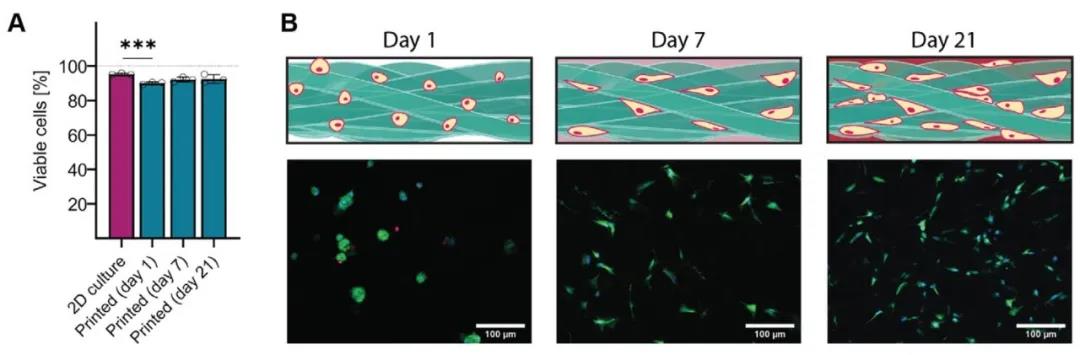
Figure 6 High cell viability 3D printing of tangled micro-bundle gel loaded chondrocytes
5. In vitro tangled micro-beam gel loaded with chondrocytes induces cartilage regeneration:
The HA-MA tangled micro-bundle gel loaded with chondrocytes was cultured for 42 days, and the gel mass was white cartilaginous, indicating dense extracellular matrix deposition. In order to confirm this, the researchers stained the cultured tissue structure for cartilage-specific markers. The staining results showed that related proteoglycans, type I collagen and type II collagen were all highly expressed. Among them, type II collagen was stained with collagen after 42 days. Enhancement, manifested as deposition in the micro-beam and deposition in the hydrogel network. Subsequently, the researchers compared the compressive modulus of chondrocytes loaded with 3D printed gel microbeams for 21 days and 42 days. The modulus increased from 2.7 ± 0.3 kPa to 212 ± 83.7 kPa, and then to 780.2 ± 218.4 kPa. Although this mechanical strength is far less than the compressive modulus of native cartilage tissue (1829.8 ± 72 kPa), it is still a significant improvement. Since the materials used in this study have very little compression resistance, the change in compressive modulus can be completely attributed to the massive deposition and maturation of the extracellular matrix (Figure 7).
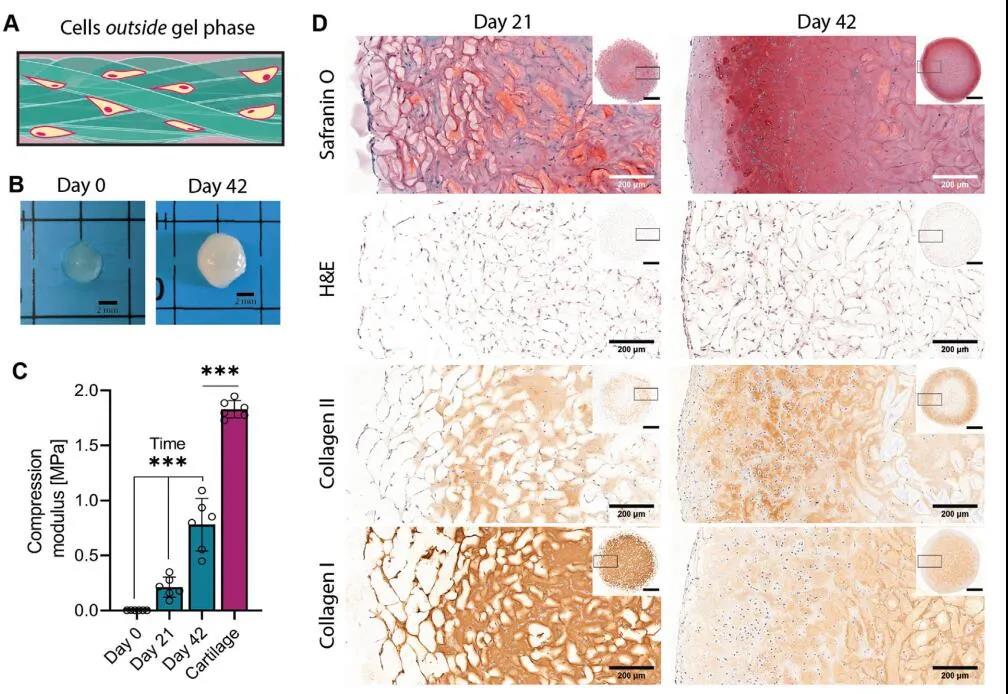
Figure 7 Tangled micro-bundle gel induces cartilage regeneration
6. Summary:
This research uses high aspect ratio hydrogel microbeams as the bio-ink. The gel microbeams in the bio-ink are entangled with each other to form a porous and stable gel network. And the tangled micro-bundle gel can promote the formation of myoblasts in myoblasts and maintain a relatively high cell activity (90%). The gel scaffold formed by co-deposition and printing of chondrocytes and gel microbeams can induce chondrocytes to form a rich extracellular matrix, and its compressive modulus is significantly improved. The construction strategy of micro-beam hydrogels in this research provides a new preparation method for 3D printing bio-inks.
Paper link:
https://doi.org/10.1002/advs.202001419
Recently, the team of Professor Marcy Zenobi-Wong from the Department of Health Science and Technology of the University of Zurich squeezed the massive hydrogel through a grid of micrometer-sized pores to decompose the hydrogel into a micro-beam gel network. The gel scaffold formed by co-deposition of chondrocytes and gel microbeams in 3D bioprinting can induce chondrocytes to form a rich extracellular matrix, and its compressive modulus is significantly improved. The construction strategy of micro-beam hydrogels in this research provides a new preparation method for 3D printing bio-inks. Related research paper: "3D Bioprinting of Macroporous Materials Based on Entangled Hydrogel Microstrands" was published on Advanced Science.

Figure 1 Schematic diagram of the construction and 3D bioprinting of entangled micro-bundle gel network
Gel material:
Methacryloyl hyaluronic acid (HA-MA), methacryloyl gelatin (GelMA), gelatin
Process:
UV cross-linked HA-MA (concentration 2% w/v, grafting rate 28%) or GelMA (concentration 2% w/v, grafting rate 92%)/gelatin block hydrogel through holes of 40~100μm The sieve is mechanically squeezed. This process decomposes the gel mass into gel micro-beams, which are randomly entangled with each other to form a structured material composed of a high aspect ratio hydrogel.
1. Stability and macro porosity of entangled micro-bundle gel:
In order to study the tunability of 3D printing entangled microbeam gels, the researchers prepared a series of HA-MA system gels with different crosslinking strengths by adjusting the UV crosslinking time (20s, 60s, 180s). In contrast, the granular hydrogel was immersed in PBS at 37°C for 7 days. The bulk microgel collapsed within 1 hour, while the entangled micro-bundle hydrogel had a long-term cohesion in an aqueous medium. In order to evaluate the porosity of the HA-MA entangled microbeam gel, the researchers immersed the gel in a high molecular weight dextran fluorescent dye. Due to the large molecular weight of dextran, Figure 2H shows that the fluorescently labeled dextran can only enter between the gel bundles and cannot penetrate the gel phase of the hydrogel. Based on this, researchers can use it to calculate the gel The porosity (Figure 2).

Figure 2 Stability and macro porosity of entangled micro-bundle gel network
2. Shear thinning and shear recovery characteristics of HA-MA entangled micro-bundle gel:
The entangled micro-beam type gel exhibits shear thinning rheological properties, which is necessary for extrusion-type 3D bioprinting. Through rheological measurement, the shear recovery rate of HA-MA microbeam gel with different crosslinking strength is different, and it changes with the difference of the sieve aperture. Through comparison, the researcher pointed out that the entanglement of medium crosslinking strength is different. The shear recovery rate of the microbeam type gel sample after initial shearing is 81.8% (mesh mesh size 40 μm) and 82% (mesh mesh size 100 μm), which is significantly better than the other two groups and can be used as an excellent 3D Print bio-ink (Figure 3).

Figure 3 Rheological properties of HA-MA entangled micro-beam gel network
3. 3D printing characteristics of entangled micro-bundle gel:
Based on the HA-MA microbeam gel, the researchers 3D bioprinted a two-layer grid structure. The higher the cross-linking rate, the more easily the gel sample breaks under a shorter stretching distance, and the higher the printing accuracy. In addition, considering the rheological properties of the entangled micro-beam type hydrogel, the entangled micro-beam type gel-like sample with medium cross-linking strength is the most excellent bio-ink. The rheological properties and printing performance of the two pore sizes (40 μm and 100 μm) are not significantly different, but the pore size has a significant effect on the porosity, so this parameter can be selected according to the actual application to adjust the macro porosity of the gel. In addition, the researchers also tested other hydrogel systems (GelMA, carrageenan, enzyme or chemically cross-linked HA, etc.). After pre-cross-linking and filtering to form a micro-beam gel, it was then subjected to extrusion-type 3D bioprinting. The test results show that all the gel systems can form entangled microfilaments and 3D print out a grid scaffold.

Figure 4 Extrusion 3D printability and anisotropy of entangled micro-bundle gel
4. 3D bioprinting of tangled micro-bundle gels loaded with cells:
The researchers used two methods to explore the cell-loading capacity of the entangled microbeam gel: Method one, first, the cells are embedded in the bulk hydrogel, and then the hydrogel is squeezed into gel microbeams; Method Second, the extruded gel microbeams are mixed with the cell suspension, so that the cells occupy the pores between the microbeams. Using method 1, the researchers chose gelatin combined with GelMA hydrogel to explore the ability of the tangled micro-bundle gel to support mouse myoblasts (C2C12). Calcein AM staining combined with Hoechst staining showed that the cells were fused and formed along the direction of microfilaments. Arrangement of multinucleated myotubes (Figure 5). Using method two, the researchers selected HA-MA hydrogel microbeams to compound with chondrocytes. The results showed that chondrocytes showed a circular phenotype after 3D bioprinting, but proliferated and displayed longer after 7 days and 21 days of culture. Shuttle type (Figure 6).

Figure 5 Tangled microbundle gel can induce myoblast formation in myoblasts

Figure 6 High cell viability 3D printing of tangled micro-bundle gel loaded chondrocytes
5. In vitro tangled micro-beam gel loaded with chondrocytes induces cartilage regeneration:
The HA-MA tangled micro-bundle gel loaded with chondrocytes was cultured for 42 days, and the gel mass was white cartilaginous, indicating dense extracellular matrix deposition. In order to confirm this, the researchers stained the cultured tissue structure for cartilage-specific markers. The staining results showed that related proteoglycans, type I collagen and type II collagen were all highly expressed. Among them, type II collagen was stained with collagen after 42 days. Enhancement, manifested as deposition in the micro-beam and deposition in the hydrogel network. Subsequently, the researchers compared the compressive modulus of chondrocytes loaded with 3D printed gel microbeams for 21 days and 42 days. The modulus increased from 2.7 ± 0.3 kPa to 212 ± 83.7 kPa, and then to 780.2 ± 218.4 kPa. Although this mechanical strength is far less than the compressive modulus of native cartilage tissue (1829.8 ± 72 kPa), it is still a significant improvement. Since the materials used in this study have very little compression resistance, the change in compressive modulus can be completely attributed to the massive deposition and maturation of the extracellular matrix (Figure 7).

Figure 7 Tangled micro-bundle gel induces cartilage regeneration
6. Summary:
This research uses high aspect ratio hydrogel microbeams as the bio-ink. The gel microbeams in the bio-ink are entangled with each other to form a porous and stable gel network. And the tangled micro-bundle gel can promote the formation of myoblasts in myoblasts and maintain a relatively high cell activity (90%). The gel scaffold formed by co-deposition and printing of chondrocytes and gel microbeams can induce chondrocytes to form a rich extracellular matrix, and its compressive modulus is significantly improved. The construction strategy of micro-beam hydrogels in this research provides a new preparation method for 3D printing bio-inks.
Paper link:
https://doi.org/10.1002/advs.202001419
18915694570
Previous: AFM: Biomimetic nanome


