Zhang Shubiao/Liang Xingjie/Zhen Yuhong ACS Nano: Temperature-sensitive lipid-coated carbon nanotubes for synergistic photothermal therapy and gene therapy
Gene therapy (GT), which uses nucleic acids to treat tumors, is considered a promising treatment strategy. In particular, small interfering RNAs (siRNAs) have attracted great attention as potential therapeutic agents because they can regulate protein expression by silencing mRNA (mRNA). Although siRNA has therapeutic potential, they still have obvious limitations, such as rapid enzymatic degradation in the body and low intracellular uptake rate, which requires improved delivery to the target. The combination of photothermal therapy (PTT) and gene therapy shows great potential for synergistic anti-tumor activity. However, the lack of a vector for gene controlled release is still a serious challenge.
In view of this, Zhang Shubiao of Dalian Nationalities University, Liang Xingjie of the National Nanoscience Center, and Zhen Yuhong of Dalian Medical University used peptide lipids (PL) and sucrose laurate (SL) to coat single-walled carbon nanotubes (SCNTs) and multi-walled carbon nanotubes. (MCNTs), forming a dual-function delivery system with excellent temperature sensitivity and photothermal performance (represented as SCNT-PS and MCNT-PS, respectively).
Key points of this article:
1) Carbon nanotubes are activated in an optical window that works at a wavelength suitable for light penetration into deep tissues. These photo-switchable gene carriers decompose as the temperature rises, releasing anti-survivin siRNA, and the siRNA escapes from the endosomes that capture them.
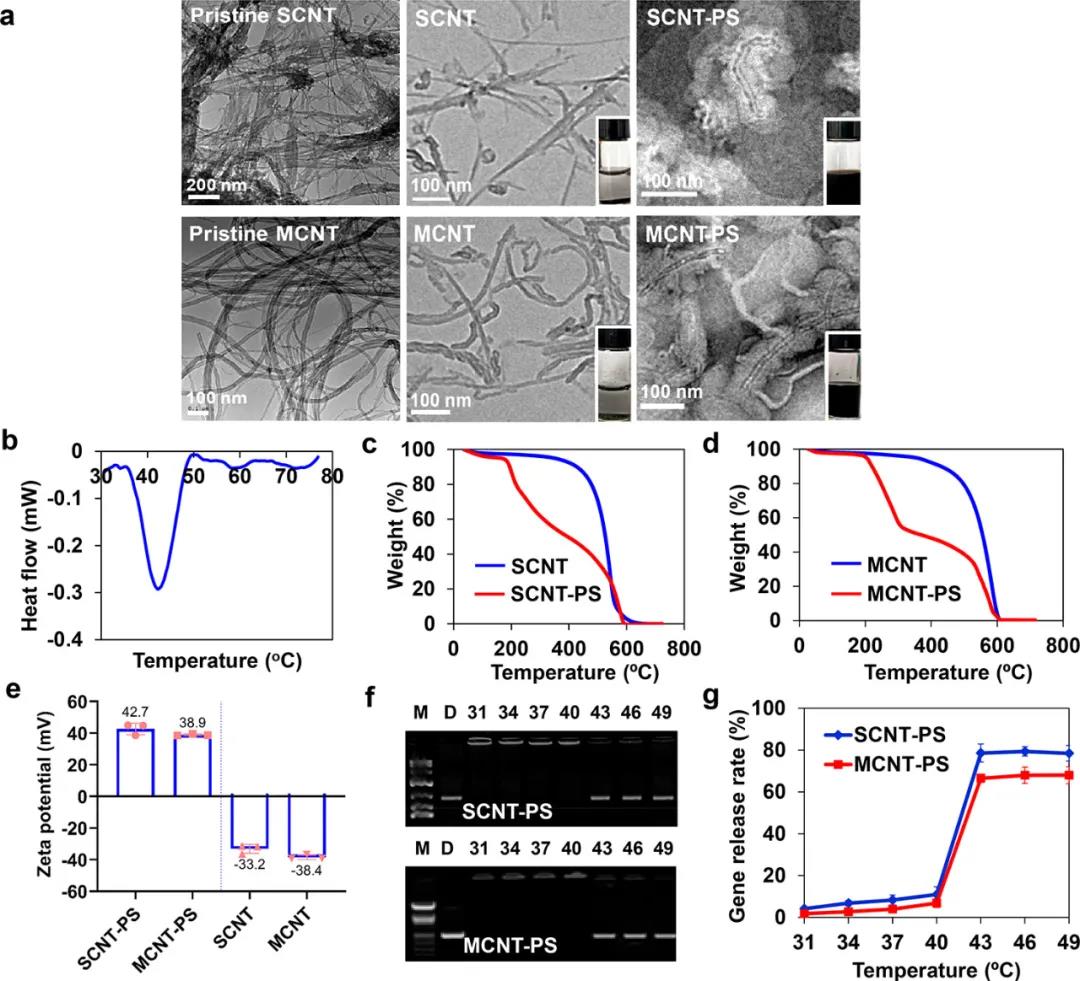
In vitro characterization of nanotubes
2) CNT/siRNA inhibits tumor growth by inhibiting the expression of survivin, and at the same time exhibits a photothermal effect under near infrared (NIR) light. SCNT-PS/siRNA has high anti-tumor activity and can completely inhibit certain tumors. Due to the effect of the PL and SL coatings, the temperature-sensitive lipid undergoes a phase change, which enables the efficient systemic delivery of siRNA to the tumor site and facilitates the release of siRNA.
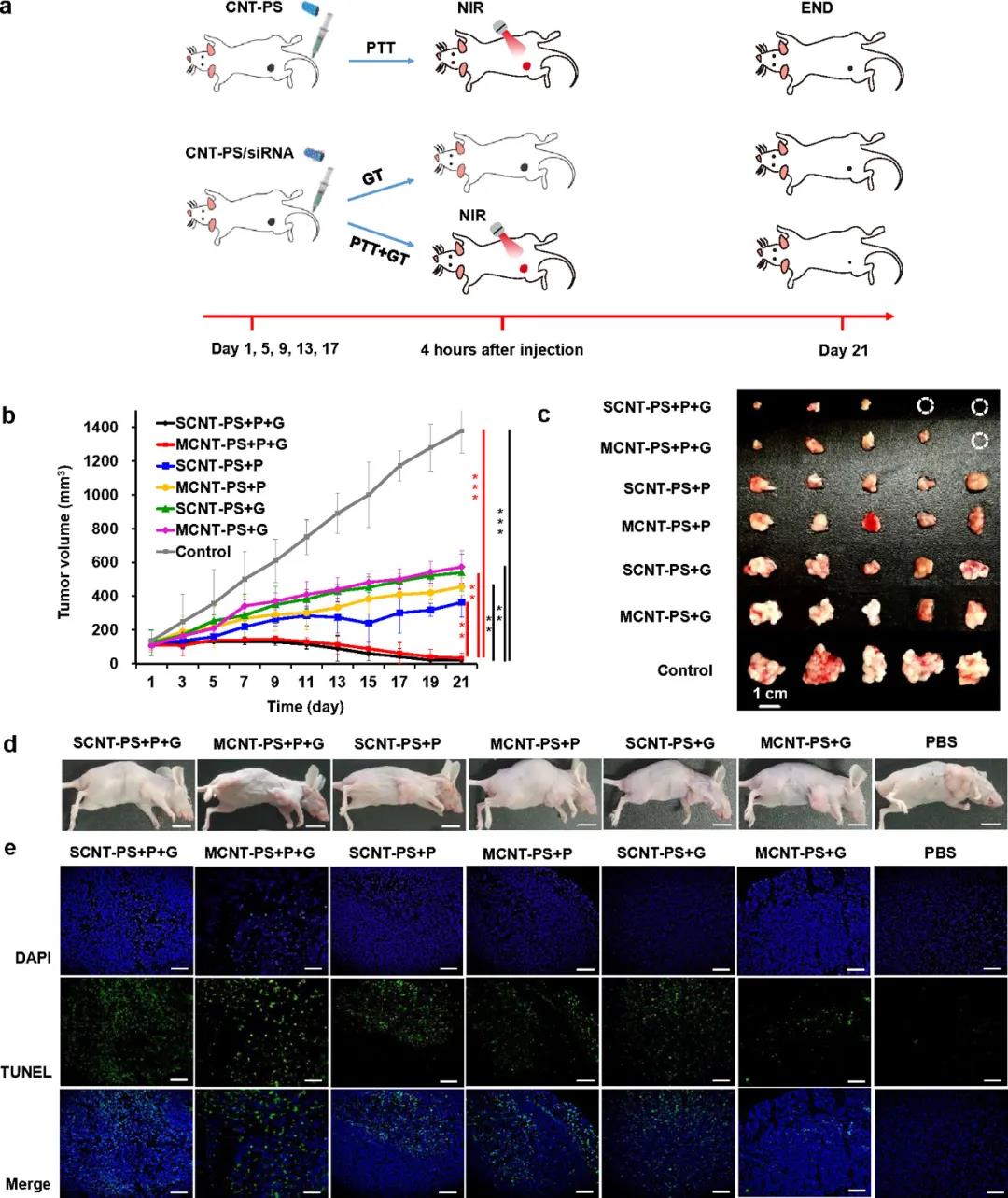
Animal tumor treatment experiment
In summary, this paper constructs bifunctional lipid-coated single-wall and multi-wall CNT delivery systems (SCNT-PS and MCNT-PS) through the self-assembly of PL and SL on CNTs. After loading therapeutic siRNA, the combination of PTT and GT produced significant tumor suppression effects in vivo and in vitro. It is worth noting that some tumors even disappeared completely after 21 days of treatment with SCNT-PS/siRNA. The researchers attributed this significant inhibition of tumor growth to the temperature sensitivity of the lipids used in the carrier. These lipids give dual-functional nanoparticles a high ability to deliver siRNA to tumor cells, as well as excellent temperature-controlled gene release inside tumor cells. In addition, SCNT-PS and MCNT-PS nanoparticles have no obvious cytotoxic effect when the concentration is as high as 60μg/mL or is non-toxic to mice. In summary, CNT-PS nanoparticles are a promising tool for future clinical cancer treatment.
references:
Yinan Zhao, et al. Temperature-Sensitive Lipid-Coated Carbon Nanotubes for Synergistic Photothermal Therapy and Gene Therapy. ACS Nano, 2021.
DOI: 10.1021/acsnano.0c08790
https://doi.org/10.1021/acsnano.0c08790
In view of this, Zhang Shubiao of Dalian Nationalities University, Liang Xingjie of the National Nanoscience Center, and Zhen Yuhong of Dalian Medical University used peptide lipids (PL) and sucrose laurate (SL) to coat single-walled carbon nanotubes (SCNTs) and multi-walled carbon nanotubes. (MCNTs), forming a dual-function delivery system with excellent temperature sensitivity and photothermal performance (represented as SCNT-PS and MCNT-PS, respectively).
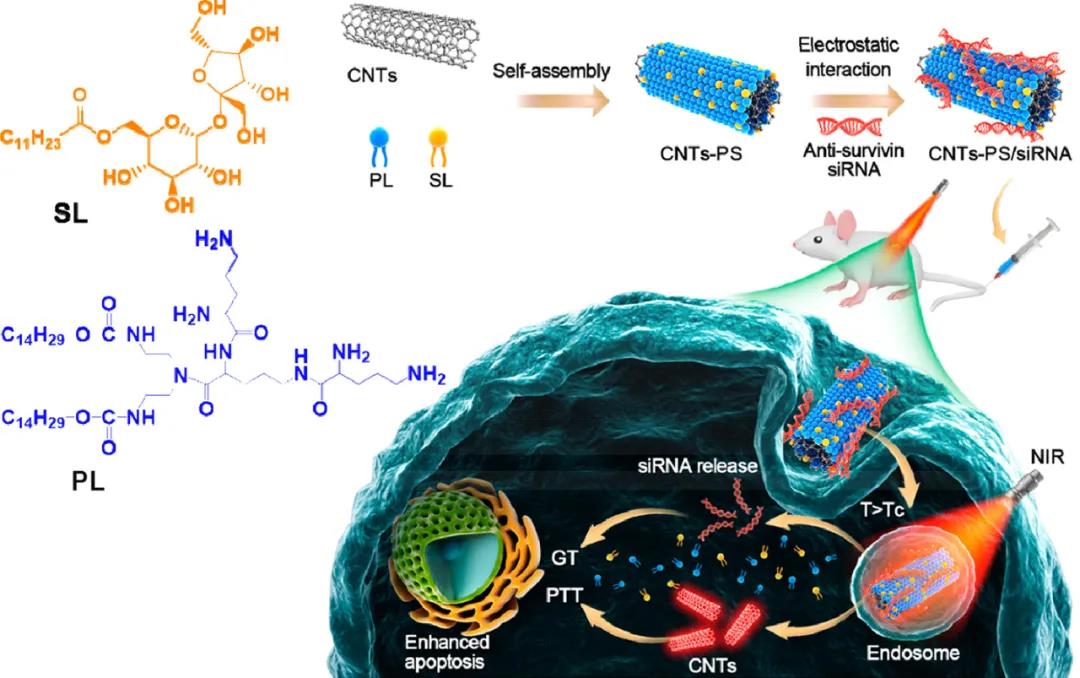
Schematic diagram
Key points of this article:
1) Carbon nanotubes are activated in an optical window that works at a wavelength suitable for light penetration into deep tissues. These photo-switchable gene carriers decompose as the temperature rises, releasing anti-survivin siRNA, and the siRNA escapes from the endosomes that capture them.

In vitro characterization of nanotubes
2) CNT/siRNA inhibits tumor growth by inhibiting the expression of survivin, and at the same time exhibits a photothermal effect under near infrared (NIR) light. SCNT-PS/siRNA has high anti-tumor activity and can completely inhibit certain tumors. Due to the effect of the PL and SL coatings, the temperature-sensitive lipid undergoes a phase change, which enables the efficient systemic delivery of siRNA to the tumor site and facilitates the release of siRNA.

Animal tumor treatment experiment
In summary, this paper constructs bifunctional lipid-coated single-wall and multi-wall CNT delivery systems (SCNT-PS and MCNT-PS) through the self-assembly of PL and SL on CNTs. After loading therapeutic siRNA, the combination of PTT and GT produced significant tumor suppression effects in vivo and in vitro. It is worth noting that some tumors even disappeared completely after 21 days of treatment with SCNT-PS/siRNA. The researchers attributed this significant inhibition of tumor growth to the temperature sensitivity of the lipids used in the carrier. These lipids give dual-functional nanoparticles a high ability to deliver siRNA to tumor cells, as well as excellent temperature-controlled gene release inside tumor cells. In addition, SCNT-PS and MCNT-PS nanoparticles have no obvious cytotoxic effect when the concentration is as high as 60μg/mL or is non-toxic to mice. In summary, CNT-PS nanoparticles are a promising tool for future clinical cancer treatment.
references:
Yinan Zhao, et al. Temperature-Sensitive Lipid-Coated Carbon Nanotubes for Synergistic Photothermal Therapy and Gene Therapy. ACS Nano, 2021.
DOI: 10.1021/acsnano.0c08790
https://doi.org/10.1021/acsnano.0c08790
18915694570
Previous: ACS Nano: Using adjust


