Tianjin University Yang Jianhai/Liu Wenguang "AM": Super soft self-fusion supramolecular polymer hydrogel, effectively preventing postoperative tissue adhesion
The intermolecular hydrogen bond density seriously affects the gelation and rheological behavior of hydrogen-bonded supramolecular polymer hydrogels, so it provides a subtle way to adjust its physical and chemical properties to meet specific biomedical applications. Recently, the team of Professor Yang Jianhai/Liu Wenguang of Tianjin University introduced a methylene spacer between the two amides in the side chain of N-acryloylglycinamide (NAGA) to generate a variant monomer N-acryloylalanineamide (NAAA).

The polymerization of NAAA in aqueous solution provides an unprecedented super-soft and highly swelling supramolecular polymer hydrogel, which is due to the weakening of the H bond caused by the additional methylene spacer. This has been achieved by variable temperature Fourier Transformation infrared (FTIR) spectroscopy and simulation calculations have been verified. Interestingly, poly(N-acryloylalanineamide) (PNAAA) hydrogel can be adjusted to form a transient network with self-fusion and excellent antifouling ability, which is due to the weak bisamide H-bond interaction and It is caused by the enhanced H-bond interaction of water amides. This self-fused PNAAA hydrogel can completely inhibit postoperative abdominal adhesions and recurrent adhesions after in vivo adhesions. This short-lived hydrogel network allows it to be broken down and excreted from the body. Molecular mechanism studies revealed the signal pathways that PNAAA hydrogel inhibits inflammation and regulates the balance of the fibrinolytic system. This self-fusion, anti-fouling ultra-soft supramolecular hydrogel is expected to be used as a barrier biological material to completely prevent postoperative tissue adhesion. Related papers were published on "AM" with the title An Ultrasoft Self-Fused Supramolecular Polymer Hydrogel for Completely Preventing Postoperative Tissue Adhesion.
Based on the subtle sensitivity of PNAGA hydrogels to molecular structure, in this work, the authors designed and synthesized a new type of N-acryloylalanine amide (NAAA) monomer, which can be used to combine alaninamide with propylene. Acid chloride (Scheme 1a). The poly(N-acryloylalanine amide) (PNAAA) hydrogel prepared by free radical polymerization of NAAA aqueous solution will have new and exciting functions. The author hypothesizes that weakened interpolymer hydrogen bonds will lead to the formation of super-soft PNAAA supramolecular hydrogels with self-fusion capabilities, while increased hydropolymer hydrogen bonds provide excellent protection against protein absorption and fibroblast adhesion. , It will be useful for postoperative anti-adhesion (Scheme 1b).
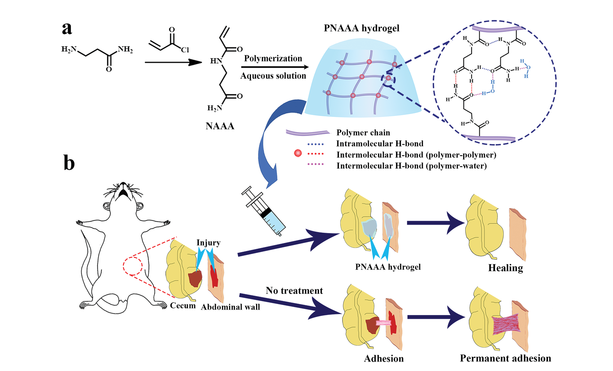
Figure 1 a, b) Schematic diagram of supramolecular PNAAA hydrogel with H-bond cross-linking network (a), and schematic diagram of rat cecal abdominal wall adhesion model with or without PNAAA hydrogel treatment (b).
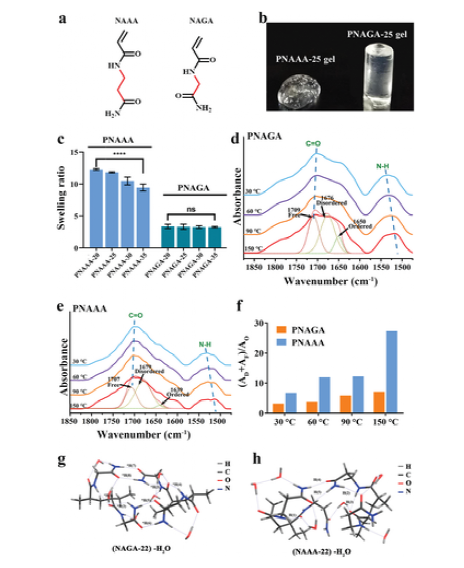
Figure 2 a) The chemical structures of N-acryloyl alaninamide (NAAA) and N-acryloyl glycinamide (NAGA). b) Photos of PNAAA-25 hydrogel and PNAGA-25 hydrogel after being soaked in normal saline for 3 days. c) The swelling rate of PNAAA hydrogel and PNAGA hydrogel with different monomer content. d, e) Variable temperature FTIR spectra of PNGA-25 hydrogel (d) and PNAAA-25 hydrogel (e); the dashed line is the curve fitting of the carbonyl group at 150°C. f) The ratio of the total amount of disordered hydrogen-bonded carbonyl groups and free carbonyl groups to the number of ordered hydrogen-bonded carbonyl groups; AF: the integral area of the fitted curve of free carbonyl groups; AD: the integral area of the fitted curve of disordered hydrogen-bonded carbonyl groups; AO :Integral area of the fitted curve of ordered hydrogen bond carbonyl group. g, h) Conformations of double NAGA dimer (NAGA-22) (g) and double NAAA dimer (NAAA-22) (h) mixed with 6H2O.
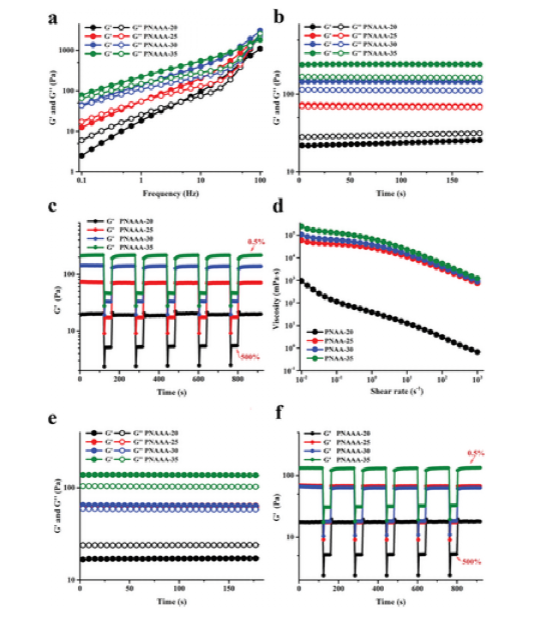
Figure 3 Rheological properties of PNAAA hydrogel. a) Sweep frequency curve (0.1-100 Hz, strain 1%, 37°C). b) Time sweep curve (0–3 minutes, 1 Hz, strain of 1%, 37°C). c) Step strain curve, 0.5% for low strain and 500% for high strain (1 Hz, 37°C). d) The shear rate scan curve is in the range of 0.01–1000 s-1. e) Time-sweeping curve of self-fusion PNAAA hydrogel (0-3 minutes, 1 Hz, strain 1%, 37°C. f) The step strain sweep of self-fusion PNAAA hydrogel is up to 500 at a low strain of 0.5% % (1 Hz, 37°C).
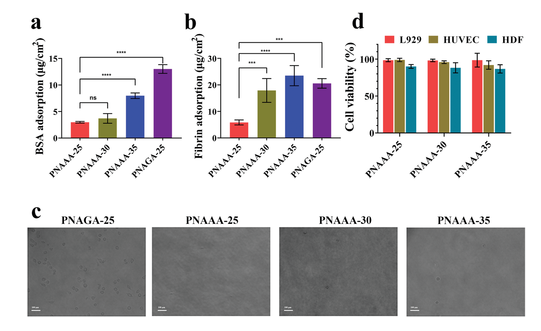
Figure 4 Antifouling ability and cell viability of self-fused PNAAA hydrogel. a) The adsorption of BSA on self-fused PNAAA hydrogel and PNAGA-25 hydrogel. b) Adsorption of fibrin on self-fused PNAAA hydrogel and PNAGA-25 hydrogel. c) After 18 hours, the cell attachment of L929 cells on the self-fused PNAAA hydrogel and PNAGA-25 hydrogel. Magnification: 200×; Scale bar: 100 μm. d) Cytotoxicity of self-fused PNAAA hydrogel.
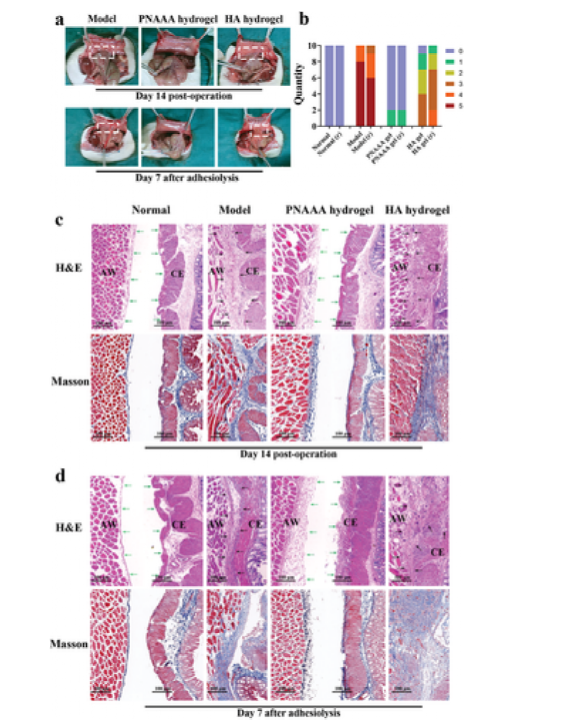
Figure 5 Postoperative anti-adhesion ability of self-fusion PNAAA hydrogel. a) Model, representative photos of abdominal adhesions in the PNAAA hydrogel and HA hydrogel groups on the 14th day after the operation and the 7th day after the adhesion. b) Adhesion scores of different groups after treatment on the 14th day after treatment and on the 7th day after adhesion dissolution. "R" means "frequently". c, d) On the 14th day after the operation and the 7th day after the adhesion, pathological analysis was performed on the specimens of the normal group, model group, PNAAA hydrogel and HA hydrogel group. CE: cecum; AW: abdominal wall.
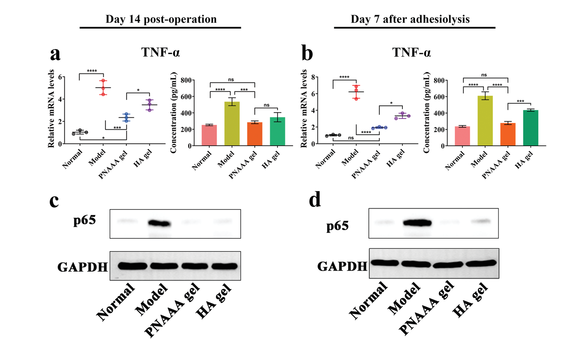
Figure 6 The effect of self-fusion PNAAA hydrogel on nuclear factor kappa B (NF-κB) signaling pathway. a, b) The relative mRNA expression of TNF-α in the injured tissue and the concentration of TNF-α in serum on the 14th day after the operation and the 7th day after the adhesion; c, d) On the 14th day after the operation and after the adhesion dissolution 7 days, the expression of p65 protein in injured tissue
Paper link:
doi.org/10.1002/adma.202008395

The polymerization of NAAA in aqueous solution provides an unprecedented super-soft and highly swelling supramolecular polymer hydrogel, which is due to the weakening of the H bond caused by the additional methylene spacer. This has been achieved by variable temperature Fourier Transformation infrared (FTIR) spectroscopy and simulation calculations have been verified. Interestingly, poly(N-acryloylalanineamide) (PNAAA) hydrogel can be adjusted to form a transient network with self-fusion and excellent antifouling ability, which is due to the weak bisamide H-bond interaction and It is caused by the enhanced H-bond interaction of water amides. This self-fused PNAAA hydrogel can completely inhibit postoperative abdominal adhesions and recurrent adhesions after in vivo adhesions. This short-lived hydrogel network allows it to be broken down and excreted from the body. Molecular mechanism studies revealed the signal pathways that PNAAA hydrogel inhibits inflammation and regulates the balance of the fibrinolytic system. This self-fusion, anti-fouling ultra-soft supramolecular hydrogel is expected to be used as a barrier biological material to completely prevent postoperative tissue adhesion. Related papers were published on "AM" with the title An Ultrasoft Self-Fused Supramolecular Polymer Hydrogel for Completely Preventing Postoperative Tissue Adhesion.
Based on the subtle sensitivity of PNAGA hydrogels to molecular structure, in this work, the authors designed and synthesized a new type of N-acryloylalanine amide (NAAA) monomer, which can be used to combine alaninamide with propylene. Acid chloride (Scheme 1a). The poly(N-acryloylalanine amide) (PNAAA) hydrogel prepared by free radical polymerization of NAAA aqueous solution will have new and exciting functions. The author hypothesizes that weakened interpolymer hydrogen bonds will lead to the formation of super-soft PNAAA supramolecular hydrogels with self-fusion capabilities, while increased hydropolymer hydrogen bonds provide excellent protection against protein absorption and fibroblast adhesion. , It will be useful for postoperative anti-adhesion (Scheme 1b).

Figure 1 a, b) Schematic diagram of supramolecular PNAAA hydrogel with H-bond cross-linking network (a), and schematic diagram of rat cecal abdominal wall adhesion model with or without PNAAA hydrogel treatment (b).

Figure 2 a) The chemical structures of N-acryloyl alaninamide (NAAA) and N-acryloyl glycinamide (NAGA). b) Photos of PNAAA-25 hydrogel and PNAGA-25 hydrogel after being soaked in normal saline for 3 days. c) The swelling rate of PNAAA hydrogel and PNAGA hydrogel with different monomer content. d, e) Variable temperature FTIR spectra of PNGA-25 hydrogel (d) and PNAAA-25 hydrogel (e); the dashed line is the curve fitting of the carbonyl group at 150°C. f) The ratio of the total amount of disordered hydrogen-bonded carbonyl groups and free carbonyl groups to the number of ordered hydrogen-bonded carbonyl groups; AF: the integral area of the fitted curve of free carbonyl groups; AD: the integral area of the fitted curve of disordered hydrogen-bonded carbonyl groups; AO :Integral area of the fitted curve of ordered hydrogen bond carbonyl group. g, h) Conformations of double NAGA dimer (NAGA-22) (g) and double NAAA dimer (NAAA-22) (h) mixed with 6H2O.

Figure 3 Rheological properties of PNAAA hydrogel. a) Sweep frequency curve (0.1-100 Hz, strain 1%, 37°C). b) Time sweep curve (0–3 minutes, 1 Hz, strain of 1%, 37°C). c) Step strain curve, 0.5% for low strain and 500% for high strain (1 Hz, 37°C). d) The shear rate scan curve is in the range of 0.01–1000 s-1. e) Time-sweeping curve of self-fusion PNAAA hydrogel (0-3 minutes, 1 Hz, strain 1%, 37°C. f) The step strain sweep of self-fusion PNAAA hydrogel is up to 500 at a low strain of 0.5% % (1 Hz, 37°C).

Figure 4 Antifouling ability and cell viability of self-fused PNAAA hydrogel. a) The adsorption of BSA on self-fused PNAAA hydrogel and PNAGA-25 hydrogel. b) Adsorption of fibrin on self-fused PNAAA hydrogel and PNAGA-25 hydrogel. c) After 18 hours, the cell attachment of L929 cells on the self-fused PNAAA hydrogel and PNAGA-25 hydrogel. Magnification: 200×; Scale bar: 100 μm. d) Cytotoxicity of self-fused PNAAA hydrogel.

Figure 5 Postoperative anti-adhesion ability of self-fusion PNAAA hydrogel. a) Model, representative photos of abdominal adhesions in the PNAAA hydrogel and HA hydrogel groups on the 14th day after the operation and the 7th day after the adhesion. b) Adhesion scores of different groups after treatment on the 14th day after treatment and on the 7th day after adhesion dissolution. "R" means "frequently". c, d) On the 14th day after the operation and the 7th day after the adhesion, pathological analysis was performed on the specimens of the normal group, model group, PNAAA hydrogel and HA hydrogel group. CE: cecum; AW: abdominal wall.

Figure 6 The effect of self-fusion PNAAA hydrogel on nuclear factor kappa B (NF-κB) signaling pathway. a, b) The relative mRNA expression of TNF-α in the injured tissue and the concentration of TNF-α in serum on the 14th day after the operation and the 7th day after the adhesion; c, d) On the 14th day after the operation and after the adhesion dissolution 7 days, the expression of p65 protein in injured tissue
Paper link:
doi.org/10.1002/adma.202008395
18915694570
Previous: Beijing University of


