China-Japan Friendship Hospital/Changchun Institute of Applied Chemistry/Peking University Dental
Malignant bone tumors are usually accompanied by bone defects and severe pathological fractures, which can complicate tumor treatment. Current treatment strategies can inhibit tumor proliferation to a large extent, but the high tumor recurrence rate and related bone defects are still a major challenge. Therefore, there is an urgent need for new biomedical materials and non-surgical strategies to prevent tumor recurrence and bone destruction.
Recently, Professor Gu Xinquan, director of the Department of Urology, Jilin University Sino-Japanese Friendship Hospital, associate researcher Wang Fan, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and Wei Yan, chief physician of Peking University Hospital of Stomatology, collaborated to develop a new type of near-infrared (NIR) light response and high mechanical strength. Hydrogel to provide excellent photothermal tumor treatment and fracture repair capabilities. Due to the up-conversion lanthanide-Au mixed nanoparticles and alginate network, the hydrogel has good biocompatibility and super photothermal effect. The subcutaneous tumor model is used to prove that the tumor can be effectively eradicated by local photothermal treatment, and there is no tumor recurrence during the observation period. In addition, the injected hydrogel has high mechanical strength and promotes the repair of bone defects by stabilizing the bone structure of the fracture. Related work was published on Advanced Functional Materials with the title "Injectable In Situ Induced Robust Hydrogel for Photothermal Therapy and Bone Fracture Repair".

【Hydrogel Design】
This research aims to develop a new type of near-infrared (NIR) light-responsive composite hydrogel (UCNP-Au-Alg) by upconverting nanoparticles (NPs) and alginate. The UCNP-Au-Alg hydrogel is rapidly heated under 808 nm NIR irradiation, which can completely eradicate the subcutaneous solid tumor in the mouse model without any recurrence. Due to the interaction with the extracellular tissue fluid Ca2+, the mechanical enhancement of the hydrogel in vivo is realized. These excellent mechanical properties enable the hydrogel to support and repair damaged bones, thus showing its potential to repair and treat bone-related complications.
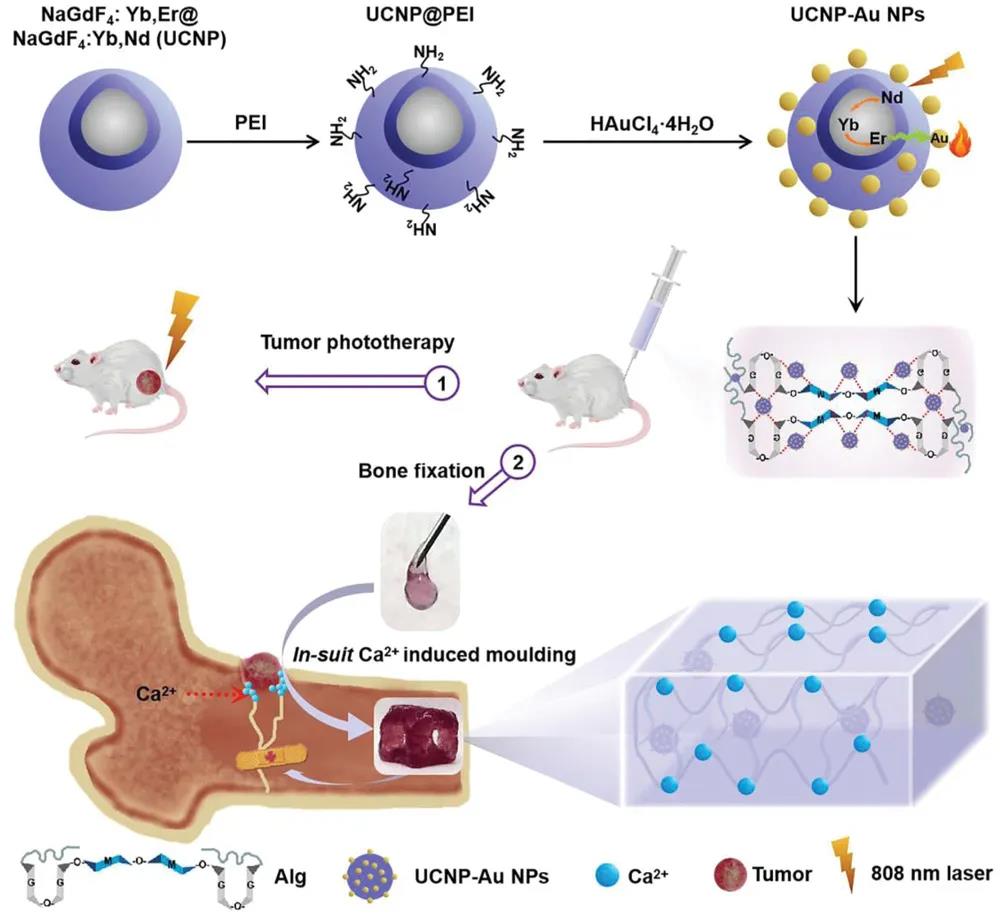
Schematic diagram of the manufacture and application of UCNP-Au-Alg hydrogel in tumor phototherapy and bone fixation
[Preparation and characterization of nanoparticles and hydrogels]
UCNP nanoparticles are prepared by solvothermal method. After the surface oleic acid is exchanged with PEI, the ultra-small Au NPs grown in situ on the surface of UCNP@PEI form UCNP-Au NPs through the heavy oxidation reaction between PEI and HAuCl. UCNP-Au aqueous solution is mixed with sodium alginate solution to obtain injectable UCNP-Au-Alg hydrogel. The high mechanical strength UCNP-Au-Alg hydrogel is formed by adding Ca2+ (2 mM) at a concentration similar to that of the extracellular matrix. The addition of calcium ions caused a major change in the morphology of the hydrogel, which in turn changed its mechanical properties. In the compression and tensile tests, the breaking strength of the hydrogel was 1.3±0.2 and 1.2±0.1 MPa, respectively.
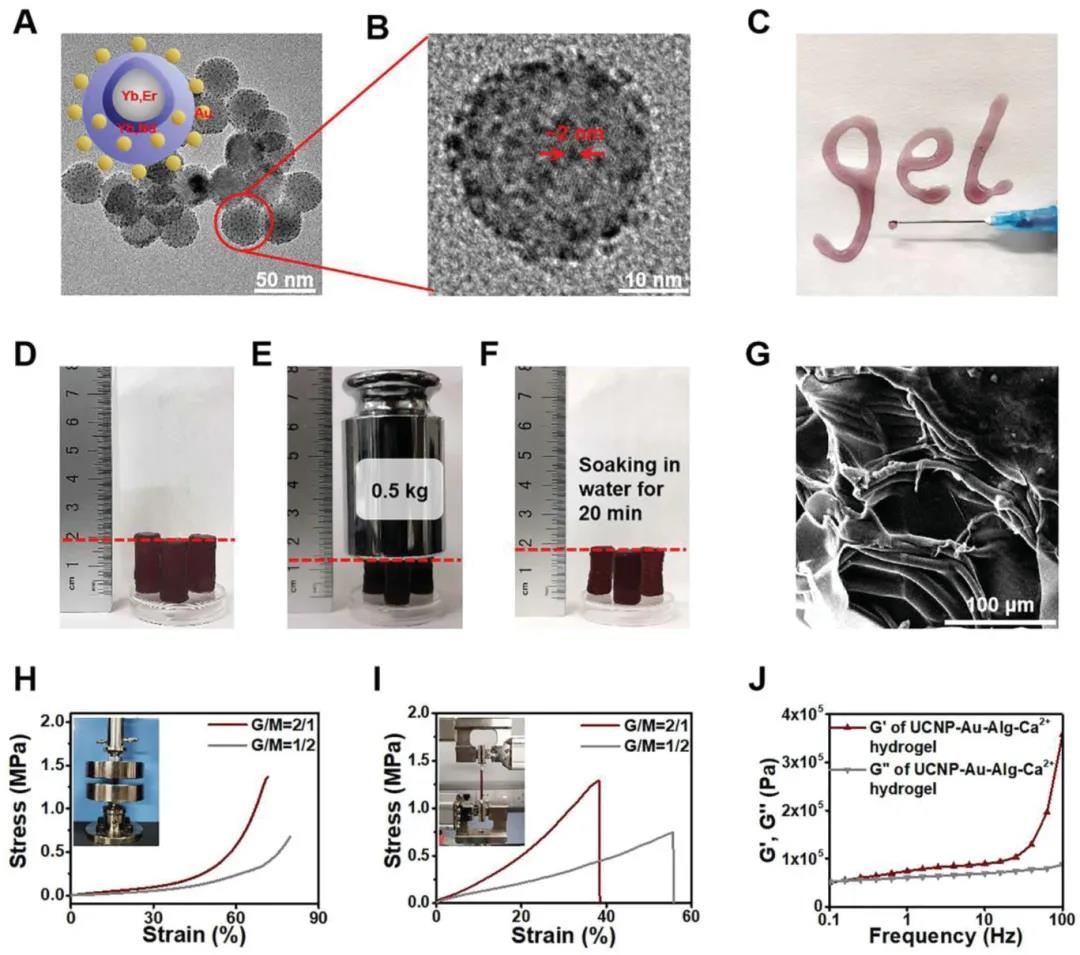
Preparation and characterization of nanoparticles and hydrogels
【UCNP-Au-Alg hydrogel in vitro photothermal effect】
UCNP-Au-Alg hydrogel exhibits excellent photothermal conversion due to the surface proton resonance of ultra-small Au NPs. Specifically, after 3 minutes of irradiation with a power density of 1 W∙cm-2, the temperature of the UCNP-Au-Alg hydrogel rapidly increased to 84°C (ΔT = 52.9 °C), and the light-to-heat conversion efficiency ( ƞ) is 36.7%. After increasing Ca2+, UCNP-Au-Alg hydrogel allows the surface temperature to reach 110 °C.
MTT and live/dead cell staining experiments show that UCNP-Au-Au-Alg hydrogel has good biocompatibility when not irradiated. After irradiation, the proliferation of T24 cells is significantly inhibited. Flow cytometry showed that 27.7% of irradiated T24 cells died of late apoptosis, which was twice the rate of necrosis caused by traditional photothermal treatment. These findings prove that UCNP-Au-Alg hydrogel has controllable NIR light-mediated cell killing ability.
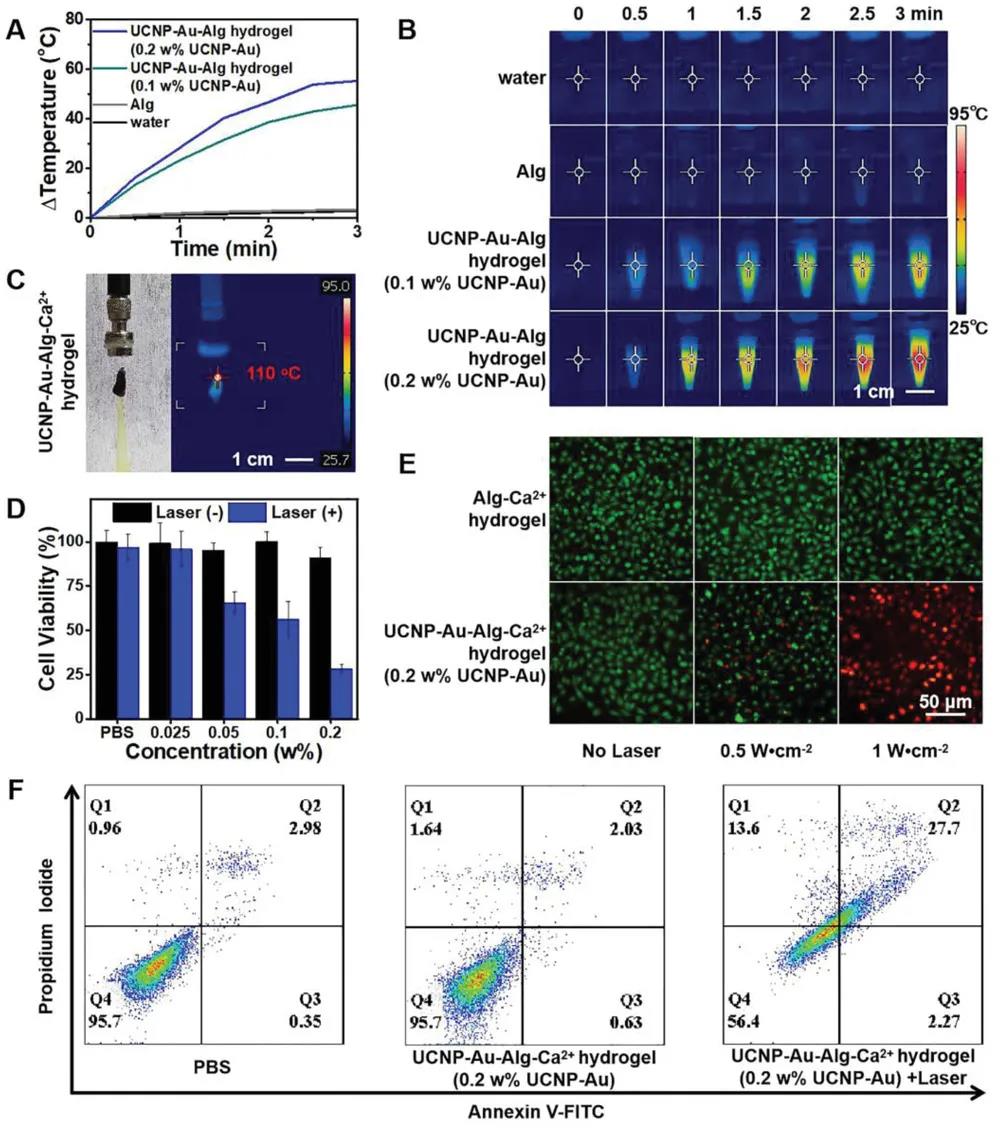
In vitro photothermal effect of UCNP-Au-Alg hydrogel
[In vivo evaluation of the light-induced anti-tumor properties and bone healing function of UCNP-Au-Alg hydrogel]
After subcutaneous injection, the hydrogel remained stable for a week. After 5 minutes, 12 hours, and one week after the injection, the local temperature of the mice increased to 55 °C after 3 minutes of irradiation with a 808 nm laser at a power density of 1 W∙cm-2.
The subcutaneous tumor and skull defect models were used to evaluate PTT and bone healing function. Female BALB/c nude mice with T24 tumors were randomly divided into five groups (n=5). Five different processing methods include: 1) PBS without laser irradiation, 2) PBS with laser irradiation, 3) UCNP-Alg-Au without laser irradiation, 4) UCNP-Alg-Au and laser irradiation once, 5) UCNP-Alg-Au and laser are irradiated twice. Compared with groups 1, 2 and 3, tumor growth in groups 4 and 5 was significantly inhibited within 14 days. In addition, the tumors in group 5 were completely eliminated after the second laser treatment, and there was no recurrence after 14 days. The staining of the tumor tissue showed that the shape of the tumor cells in the control group was irregular. In contrast, most tumor cells treated with hydrogel plus irradiation have shrunk, and significant apoptosis has been observed.
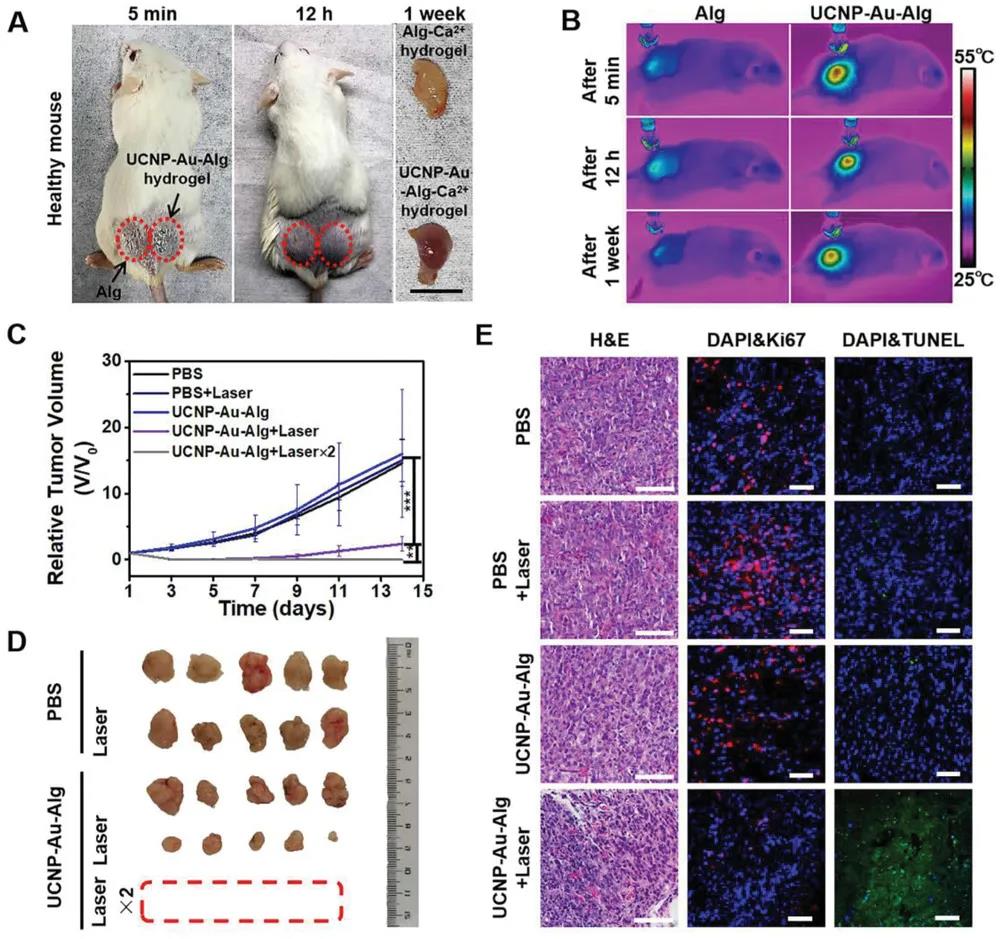
In vivo evaluation of the light-induced anti-tumor properties of UCNP-Au-Alg hydrogel
In addition to its photothermal treatment effect, after the bone defect was modeled, due to the fixing ability of the strong hydrogel, no fracture was observed in the skull treated with UCNP-Au-Alg hydrogel, and no fracture was formed during the healing process. Break line.
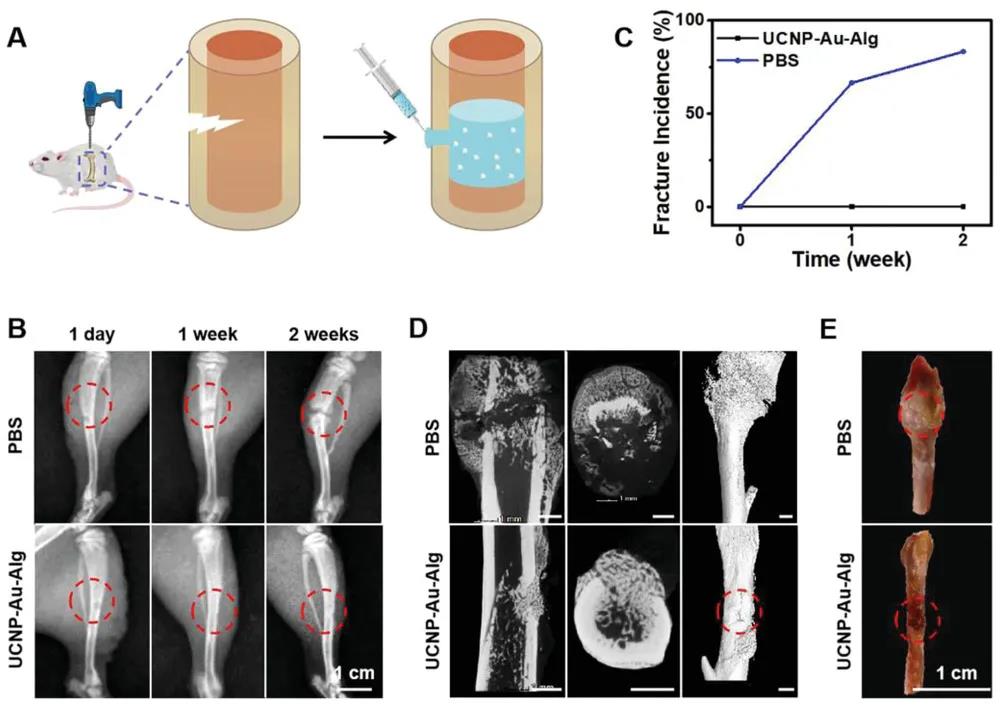
In vivo model to evaluate the bone defect repair and fixation capabilities of UCNP-Au-Alg hydrogel
Summary: This article has developed a new type of sturdy UCNP-Au-Alg composite hydrogel with excellent light-to-heat conversion and retention properties. The subcutaneous tumor model is used to prove that the tumor can be completely eradicated by non-invasive NIR light manipulation. The self-adaptive mechanical enhancement of the soft material is achieved by the interaction between the body and the environment Ca2+, which promotes the effective healing of damaged bones. Therefore, this single material shows promise as a bone-related cancer treatment and minimizes pathological rupture caused by the influence of internal and external pressure. UCNP-Au-Alg hydrogel may be a new method for clinical application of bone-related metastatic tumors.
Full text link:
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202010779
Recently, Professor Gu Xinquan, director of the Department of Urology, Jilin University Sino-Japanese Friendship Hospital, associate researcher Wang Fan, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and Wei Yan, chief physician of Peking University Hospital of Stomatology, collaborated to develop a new type of near-infrared (NIR) light response and high mechanical strength. Hydrogel to provide excellent photothermal tumor treatment and fracture repair capabilities. Due to the up-conversion lanthanide-Au mixed nanoparticles and alginate network, the hydrogel has good biocompatibility and super photothermal effect. The subcutaneous tumor model is used to prove that the tumor can be effectively eradicated by local photothermal treatment, and there is no tumor recurrence during the observation period. In addition, the injected hydrogel has high mechanical strength and promotes the repair of bone defects by stabilizing the bone structure of the fracture. Related work was published on Advanced Functional Materials with the title "Injectable In Situ Induced Robust Hydrogel for Photothermal Therapy and Bone Fracture Repair".

【Hydrogel Design】
This research aims to develop a new type of near-infrared (NIR) light-responsive composite hydrogel (UCNP-Au-Alg) by upconverting nanoparticles (NPs) and alginate. The UCNP-Au-Alg hydrogel is rapidly heated under 808 nm NIR irradiation, which can completely eradicate the subcutaneous solid tumor in the mouse model without any recurrence. Due to the interaction with the extracellular tissue fluid Ca2+, the mechanical enhancement of the hydrogel in vivo is realized. These excellent mechanical properties enable the hydrogel to support and repair damaged bones, thus showing its potential to repair and treat bone-related complications.

Schematic diagram of the manufacture and application of UCNP-Au-Alg hydrogel in tumor phototherapy and bone fixation
[Preparation and characterization of nanoparticles and hydrogels]
UCNP nanoparticles are prepared by solvothermal method. After the surface oleic acid is exchanged with PEI, the ultra-small Au NPs grown in situ on the surface of UCNP@PEI form UCNP-Au NPs through the heavy oxidation reaction between PEI and HAuCl. UCNP-Au aqueous solution is mixed with sodium alginate solution to obtain injectable UCNP-Au-Alg hydrogel. The high mechanical strength UCNP-Au-Alg hydrogel is formed by adding Ca2+ (2 mM) at a concentration similar to that of the extracellular matrix. The addition of calcium ions caused a major change in the morphology of the hydrogel, which in turn changed its mechanical properties. In the compression and tensile tests, the breaking strength of the hydrogel was 1.3±0.2 and 1.2±0.1 MPa, respectively.

Preparation and characterization of nanoparticles and hydrogels
【UCNP-Au-Alg hydrogel in vitro photothermal effect】
UCNP-Au-Alg hydrogel exhibits excellent photothermal conversion due to the surface proton resonance of ultra-small Au NPs. Specifically, after 3 minutes of irradiation with a power density of 1 W∙cm-2, the temperature of the UCNP-Au-Alg hydrogel rapidly increased to 84°C (ΔT = 52.9 °C), and the light-to-heat conversion efficiency ( ƞ) is 36.7%. After increasing Ca2+, UCNP-Au-Alg hydrogel allows the surface temperature to reach 110 °C.
MTT and live/dead cell staining experiments show that UCNP-Au-Au-Alg hydrogel has good biocompatibility when not irradiated. After irradiation, the proliferation of T24 cells is significantly inhibited. Flow cytometry showed that 27.7% of irradiated T24 cells died of late apoptosis, which was twice the rate of necrosis caused by traditional photothermal treatment. These findings prove that UCNP-Au-Alg hydrogel has controllable NIR light-mediated cell killing ability.

In vitro photothermal effect of UCNP-Au-Alg hydrogel
[In vivo evaluation of the light-induced anti-tumor properties and bone healing function of UCNP-Au-Alg hydrogel]
After subcutaneous injection, the hydrogel remained stable for a week. After 5 minutes, 12 hours, and one week after the injection, the local temperature of the mice increased to 55 °C after 3 minutes of irradiation with a 808 nm laser at a power density of 1 W∙cm-2.
The subcutaneous tumor and skull defect models were used to evaluate PTT and bone healing function. Female BALB/c nude mice with T24 tumors were randomly divided into five groups (n=5). Five different processing methods include: 1) PBS without laser irradiation, 2) PBS with laser irradiation, 3) UCNP-Alg-Au without laser irradiation, 4) UCNP-Alg-Au and laser irradiation once, 5) UCNP-Alg-Au and laser are irradiated twice. Compared with groups 1, 2 and 3, tumor growth in groups 4 and 5 was significantly inhibited within 14 days. In addition, the tumors in group 5 were completely eliminated after the second laser treatment, and there was no recurrence after 14 days. The staining of the tumor tissue showed that the shape of the tumor cells in the control group was irregular. In contrast, most tumor cells treated with hydrogel plus irradiation have shrunk, and significant apoptosis has been observed.

In vivo evaluation of the light-induced anti-tumor properties of UCNP-Au-Alg hydrogel
In addition to its photothermal treatment effect, after the bone defect was modeled, due to the fixing ability of the strong hydrogel, no fracture was observed in the skull treated with UCNP-Au-Alg hydrogel, and no fracture was formed during the healing process. Break line.

In vivo model to evaluate the bone defect repair and fixation capabilities of UCNP-Au-Alg hydrogel
Summary: This article has developed a new type of sturdy UCNP-Au-Alg composite hydrogel with excellent light-to-heat conversion and retention properties. The subcutaneous tumor model is used to prove that the tumor can be completely eradicated by non-invasive NIR light manipulation. The self-adaptive mechanical enhancement of the soft material is achieved by the interaction between the body and the environment Ca2+, which promotes the effective healing of damaged bones. Therefore, this single material shows promise as a bone-related cancer treatment and minimizes pathological rupture caused by the influence of internal and external pressure. UCNP-Au-Alg hydrogel may be a new method for clinical application of bone-related metastatic tumors.
Full text link:
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202010779
18915694570
Previous: Angew: New member! Mec


