Insulin-loaded micellar compound injectable hydrogel for diabetic wound repair
Due to susceptibility to drug-resistant bacterial infections, diabetic wound healing is very difficult. At present, a variety of antibacterial agents including metals and carbon nanomaterials (gold, copper oxide, zinc oxide, carbon nanotubes and graphene) have emerged as antibiotic substitutes in related research fields. However, the application of these nanomaterials is limited due to their inability to biodegrade or their poor cell compatibility. In addition to bacterial infections, hyperglycemia and oxidative microenvironment further hinder the recovery of diabetic skin wounds. In diabetic conditions, immune cells, including neutrophils, produce more reactive oxygen species (ROS), which can lead to prolonged scar formation or wounds that cannot heal.
In order to cope with the problems of bacterial infection, high oxidation and high blood sugar in diabetic wounds, Professor Zhang Qiuyu's team from Northwestern Polytechnical University developed an injectable water based on aldehyde modified F127 micelles/polylysine coated manganese dioxide Gel (FEM hydrogel). The polylysine in the hydrogel system has antibacterial properties, the reducing properties of manganese dioxide are used to neutralize oxidizing substances, and F127 can contain insulin for lowering blood sugar. Related research paper: Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multidrug Resistant Bacteria was published in the journal Nano Letters.
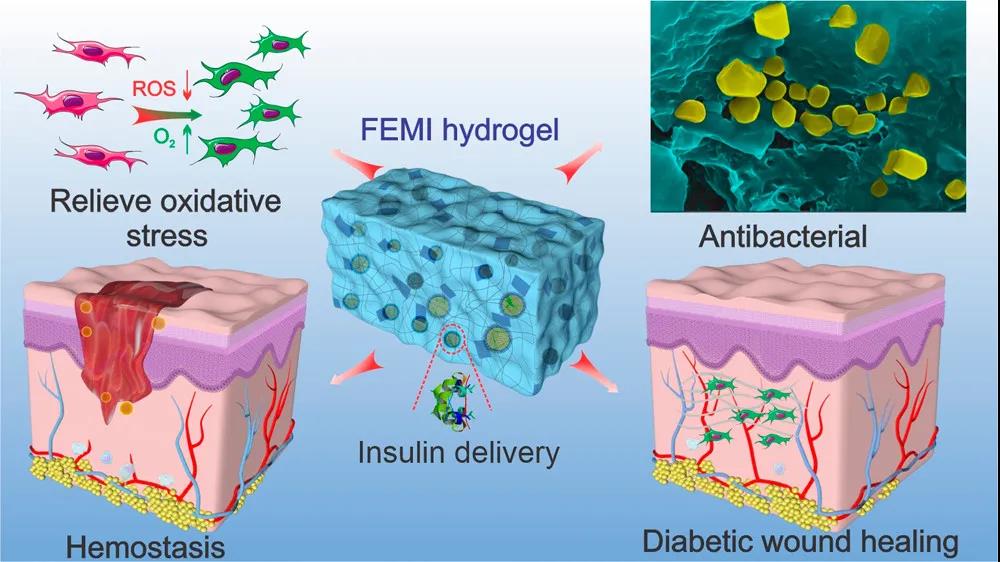
Figure 1 Schematic diagram of the antibacterial, antioxidant, and hypoglycemic multifunctional injectable hydrogel to promote diabetic wound repair
1. The construction of hydrogel system. Researchers modified F127 molecules by aldehyde grouping, so that the micelles formed on the surface are rich in aldehyde groups. The micelles rich in aldehyde groups can be combined with polylysine rich in amino groups. Through the Schiff base curing and cross-linking, at the same time F127 can be thermally gelled. This dual gel characteristic makes it have good injectability. The hypoglycemic drug insulin is coated in the hydrophobic core of F127 micelles, and the manganese dioxide is coated with polylysine at the same time, thereby obtaining a hydrocoagulable dressing with antibacterial, anti-oxidation and hypoglycemic properties. Because polylysine has cell adhesion properties, the hydrogel can well support cell growth and facilitate wound tissue healing. (figure 2)
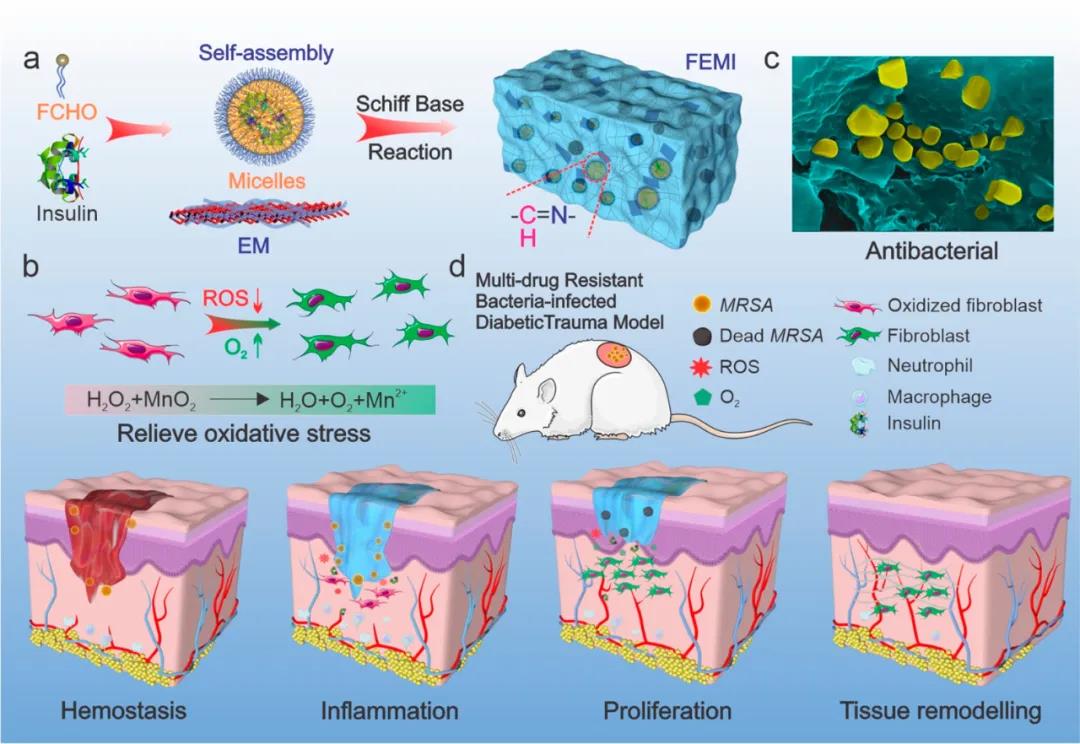
Figure 2 Schematic diagram of FEM hydrogel preparation
2. FEM dressing has continuous antibacterial and free radical elimination ability. Elevated glucose in diabetic patients participates in the mass production of ROS, which hinders wound healing. The addition of manganese dioxide can effectively eliminate ROS and supplement oxygen. Compared with the hydrogen peroxide control group, fibroblasts co-incubated with FEM-4 hydrogel showed significantly lower green fluorescence, indicating that FEM hydrogel can effectively alleviate intracellular oxidative stress. (Figure 3e)
Researchers have studied the insulin release behavior of FEM hydrogel (Figure 3 ⅰ). Compared with the control group, under acidic and oxidative conditions, insulin is released rapidly within the first 6 hours, showing a unique pH/redox dual responsiveness.
In addition, the cytotoxicity of FEM hydrogel and different components was studied. After 24 and 48 hours of incubation, both fibroblasts and myoblasts showed high cell viability.
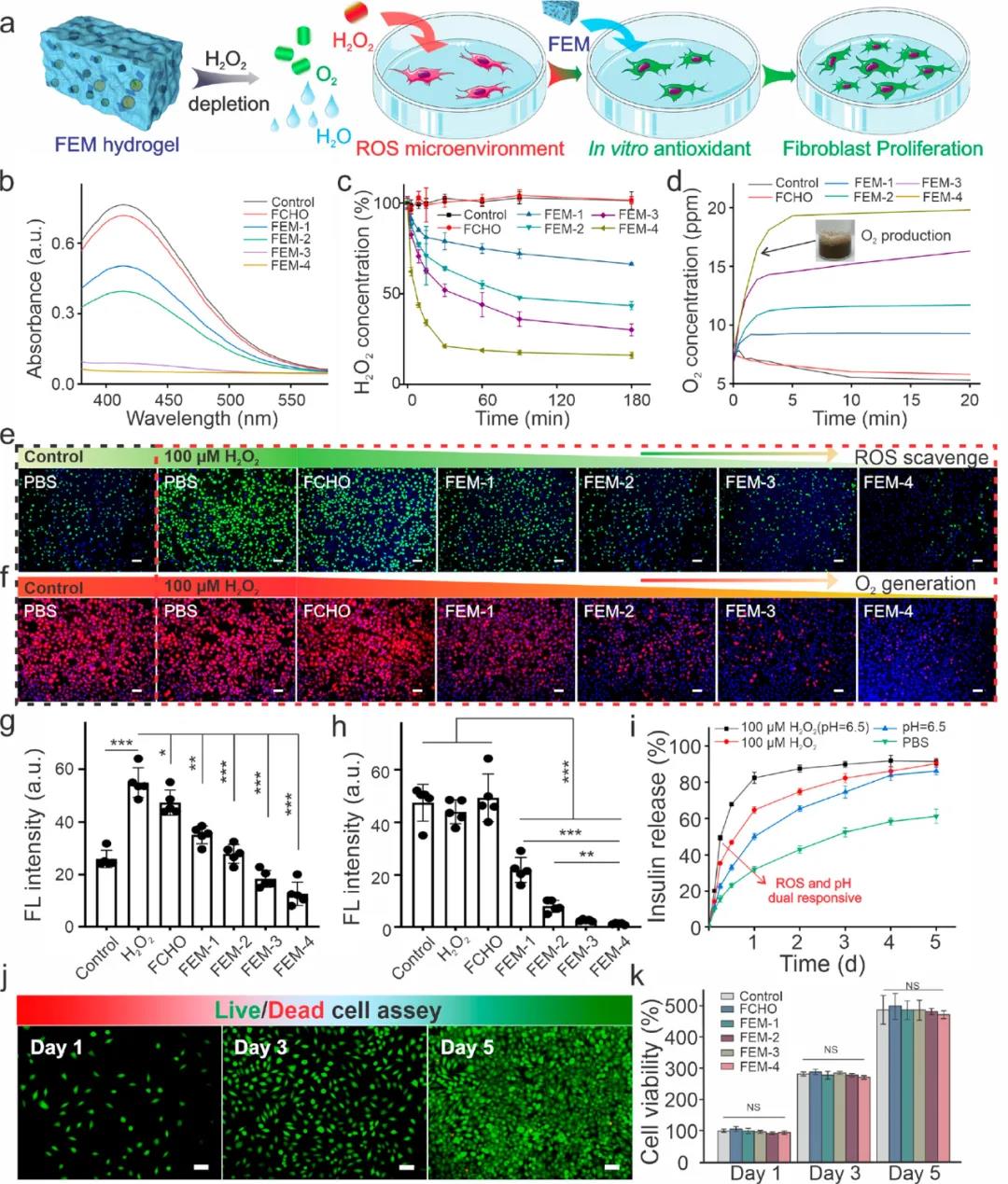
Figure 3 Antioxidant, insulin release and biocompatibility test of FEM hydrogel
3. The antibacterial properties of FEM hydrogels. The in vitro evaluation results of the antibacterial behaviors of FEM hydrogels show that: compared with PBS and antibiotic ampicillin, FEM hydrogels show better performance against Staphylococcus aureus, Escherichia coli and MRSA Antibacterial ability. In addition, with the increase in the content of the coated manganese dioxide flakes in the FEM hydrogel, the antibacterial effect was significantly enhanced. Among them, the FEM-4 group showed the best antibacterial ability, and the bacterial colonies were reduced by almost 100% (Figure 4a).
A mouse hemorrhagic liver model was used to evaluate the hemostatic ability of FEM hydrogel (Figure 4d). Compared with the blank control group, the blood loss of the FEM group was significantly reduced (Figure 4e, f). The fast hemostatic properties of FEM hydrogel may be attributed to the short gel time and strong tissue adhesion. In addition, the positively charged amino groups on the surface of the hydrogel can also bind tightly to blood cells through electrostatic interactions, thereby absorbing cells and activating the formation of blood clots.
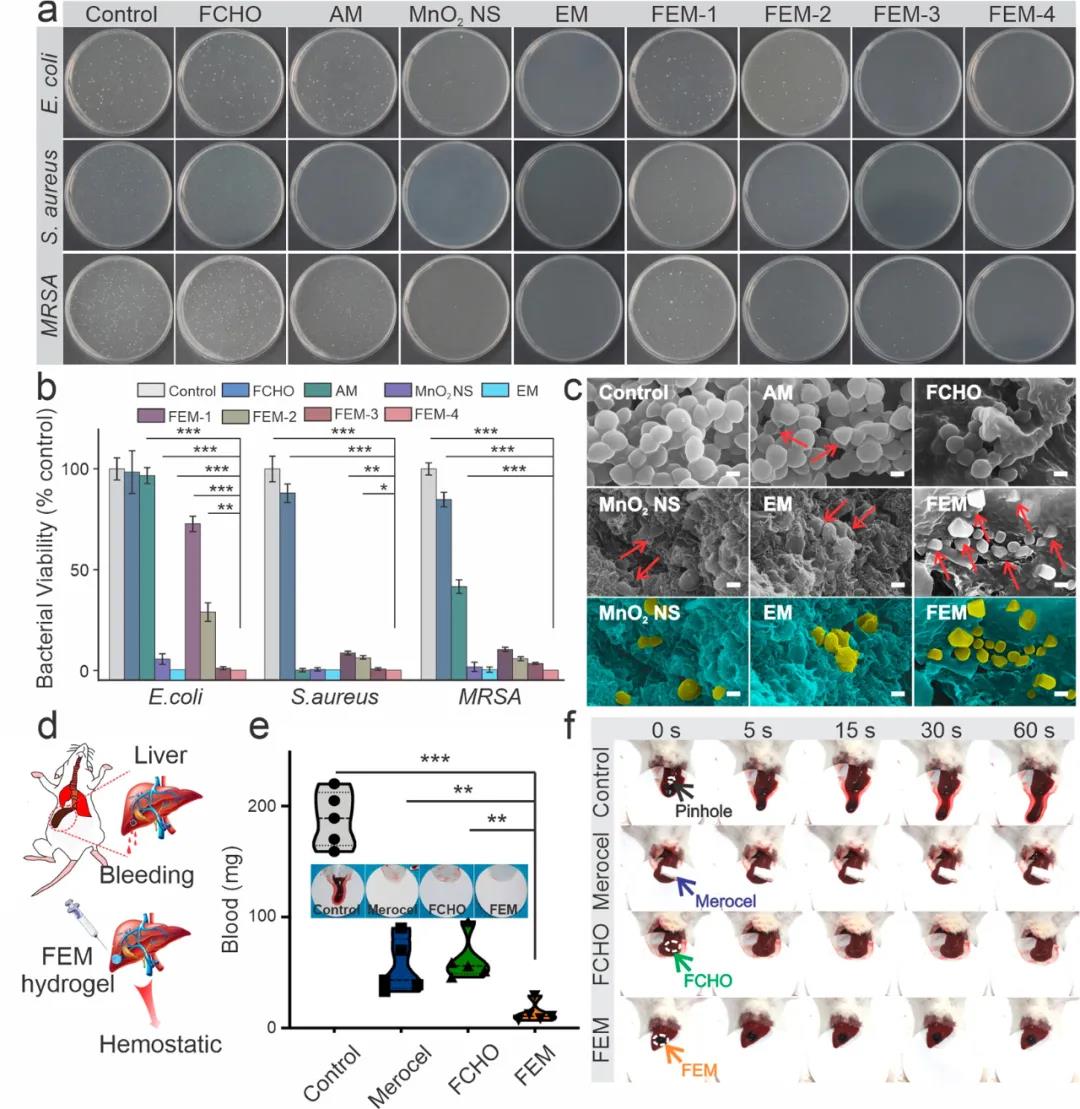
Figure 4 Evaluation of antibacterial and hemostatic properties of FEM hydrogel
4. Evaluation of the wound model of GelTA-Alla dressing. The hydrogel was injected directly into the wound on the back of the mouse. Compared with the control group on day 3, the FEM and FEMI (loaded insulin) hydrogel groups both showed the most effective bactericidal effects. In continuous wound healing experiments, the wounds of mice treated with FEM and FEMI showed significantly accelerated wound closure. Wounds with FEMI hydrogel healed best on day 14 (11.70% remaining) and were basically covered by newly formed skin (Figure 5b–d).
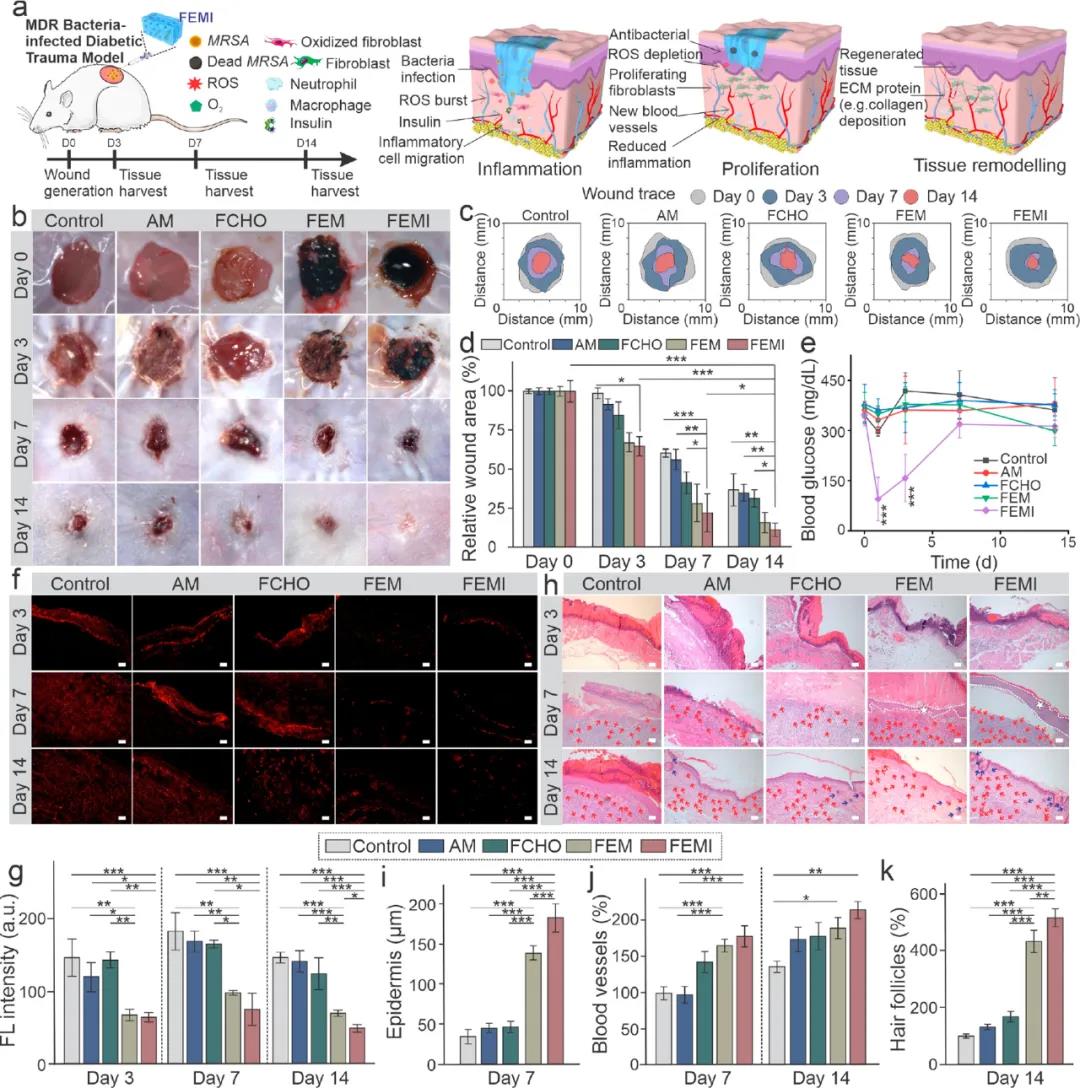
Figure 5 FEM hydrogel evaluation of animal model of sugar disease wound
The study designed an anti-oxidant, anti-bacterial and hypoglycemic injectable hydrogel dressing, and verified that it can effectively promote the healing of bacterially infected diabetic wounds. The hydrogel dressing can also synergistically reduce inflammation, stimulate angiogenesis, accelerate cell proliferation, promote granulation tissue formation and ECM deposition, and show great potential in accelerating diabetic skin reconstruction in vivo.
In order to cope with the problems of bacterial infection, high oxidation and high blood sugar in diabetic wounds, Professor Zhang Qiuyu's team from Northwestern Polytechnical University developed an injectable water based on aldehyde modified F127 micelles/polylysine coated manganese dioxide Gel (FEM hydrogel). The polylysine in the hydrogel system has antibacterial properties, the reducing properties of manganese dioxide are used to neutralize oxidizing substances, and F127 can contain insulin for lowering blood sugar. Related research paper: Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multidrug Resistant Bacteria was published in the journal Nano Letters.

Figure 1 Schematic diagram of the antibacterial, antioxidant, and hypoglycemic multifunctional injectable hydrogel to promote diabetic wound repair
1. The construction of hydrogel system. Researchers modified F127 molecules by aldehyde grouping, so that the micelles formed on the surface are rich in aldehyde groups. The micelles rich in aldehyde groups can be combined with polylysine rich in amino groups. Through the Schiff base curing and cross-linking, at the same time F127 can be thermally gelled. This dual gel characteristic makes it have good injectability. The hypoglycemic drug insulin is coated in the hydrophobic core of F127 micelles, and the manganese dioxide is coated with polylysine at the same time, thereby obtaining a hydrocoagulable dressing with antibacterial, anti-oxidation and hypoglycemic properties. Because polylysine has cell adhesion properties, the hydrogel can well support cell growth and facilitate wound tissue healing. (figure 2)

Figure 2 Schematic diagram of FEM hydrogel preparation
2. FEM dressing has continuous antibacterial and free radical elimination ability. Elevated glucose in diabetic patients participates in the mass production of ROS, which hinders wound healing. The addition of manganese dioxide can effectively eliminate ROS and supplement oxygen. Compared with the hydrogen peroxide control group, fibroblasts co-incubated with FEM-4 hydrogel showed significantly lower green fluorescence, indicating that FEM hydrogel can effectively alleviate intracellular oxidative stress. (Figure 3e)
Researchers have studied the insulin release behavior of FEM hydrogel (Figure 3 ⅰ). Compared with the control group, under acidic and oxidative conditions, insulin is released rapidly within the first 6 hours, showing a unique pH/redox dual responsiveness.
In addition, the cytotoxicity of FEM hydrogel and different components was studied. After 24 and 48 hours of incubation, both fibroblasts and myoblasts showed high cell viability.

Figure 3 Antioxidant, insulin release and biocompatibility test of FEM hydrogel
3. The antibacterial properties of FEM hydrogels. The in vitro evaluation results of the antibacterial behaviors of FEM hydrogels show that: compared with PBS and antibiotic ampicillin, FEM hydrogels show better performance against Staphylococcus aureus, Escherichia coli and MRSA Antibacterial ability. In addition, with the increase in the content of the coated manganese dioxide flakes in the FEM hydrogel, the antibacterial effect was significantly enhanced. Among them, the FEM-4 group showed the best antibacterial ability, and the bacterial colonies were reduced by almost 100% (Figure 4a).
A mouse hemorrhagic liver model was used to evaluate the hemostatic ability of FEM hydrogel (Figure 4d). Compared with the blank control group, the blood loss of the FEM group was significantly reduced (Figure 4e, f). The fast hemostatic properties of FEM hydrogel may be attributed to the short gel time and strong tissue adhesion. In addition, the positively charged amino groups on the surface of the hydrogel can also bind tightly to blood cells through electrostatic interactions, thereby absorbing cells and activating the formation of blood clots.

Figure 4 Evaluation of antibacterial and hemostatic properties of FEM hydrogel
4. Evaluation of the wound model of GelTA-Alla dressing. The hydrogel was injected directly into the wound on the back of the mouse. Compared with the control group on day 3, the FEM and FEMI (loaded insulin) hydrogel groups both showed the most effective bactericidal effects. In continuous wound healing experiments, the wounds of mice treated with FEM and FEMI showed significantly accelerated wound closure. Wounds with FEMI hydrogel healed best on day 14 (11.70% remaining) and were basically covered by newly formed skin (Figure 5b–d).

Figure 5 FEM hydrogel evaluation of animal model of sugar disease wound
The study designed an anti-oxidant, anti-bacterial and hypoglycemic injectable hydrogel dressing, and verified that it can effectively promote the healing of bacterially infected diabetic wounds. The hydrogel dressing can also synergistically reduce inflammation, stimulate angiogenesis, accelerate cell proliferation, promote granulation tissue formation and ECM deposition, and show great potential in accelerating diabetic skin reconstruction in vivo.
18915694570
Previous: Qian Zhiyong Small. Me


