Southern Medical University Yingfeng Tu "Advanced Science": A bionic nano-platform with near-infrared light response for photothermal and chemotherapy synergistic treatment
Recently, the team of Professor Yingfeng Tu from Southern Medical University published a research paper entitled "Hyperthermia-Triggered On-Demand Biomimetic Nanocarriers for Synergetic Photothermal and Chemotherapy" in the international academic journal Advanced Science (IF=15.804), and was selected as the current Frontispiece. This article mainly develops a bionic nano-platform with near-infrared light response for synergistic photothermal and chemotherapy.

In the field of tumor therapy, nanoparticles have attracted widespread attention due to their unique size effect. However, the passive targeting effect of nanoparticles is relatively limited, especially for the delivery of a single chemotherapeutic drug, which tends to cause systemic side effects and has a high tumor recurrence rate. In recent years, people have gradually developed a variety of tumor treatment methods with different characteristics based on chemotherapy, such as photothermal therapy, photodynamic therapy, starvation therapy, etc. However, these single therapies have certain shortcomings that affect the efficacy, and multi-modal collaborative treatment is expected to revolutionize the dilemma faced by tumor treatment.

The team of Professor Yingfeng Tu and the team of Peng Fei of Sun Yat-sen University and the team of Jan van Hest of Eindhoven University of Technology in the Netherlands have designed a multifunctional bionic nanosystem based on gold nanorods. Inspired by the ion chelation between cisplatin and carboxyl groups on hyaluronic acid, the researchers modified the surface of gold nanorods with mercapto-hyaluronic acid, and successfully crosslinked the surface of gold nanorods to form a gel carrier. The drug storehouse enables the classic anticancer drugs doxorubicin and cisplatin to be loaded simultaneously. In addition, in order to achieve a better tumor targeting effect, this research wraps the tumor cell membrane on the surface of the nanocarrier, and finally obtains the biomimetic nanosystem. When co-incubated with homologous and non-homologous tumor cells, the biomimetic nanocarrier can be selectively enriched in homologous tumor cells and exhibits excellent tumor targeting ability. After 24 hours of tail vein injection, the vector achieved a large amount of enrichment in the mouse transplanted tumor. Under the irradiation of the near-infrared laser, the gold nanorods in the carrier core can produce a photothermal effect, and the local thermal energy triggers the release of the loaded chemotherapeutic drugs, thereby achieving coordinated photothermal and chemotherapy for the tumor.
The first author and the last corresponding author of the research paper are both School of Pharmacy, Southern Medical University. The first author of the paper is Gao Junbin, a graduate student of the School of Pharmacy in 2018, and the last corresponding author is Professor Tu Yingfeng; Professor Peng Fei of Sun Yat-sen University Professor Jan van Hest from Eindhoven University of Technology in the Netherlands is the co-corresponding author. The research was supported by the Overseas Youth Project of the Central Organization Department, the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province, and the Pearl River Scholars.
In addition, Professor Tu Yingfeng's team has made new research progress in the field of micro-nanomotor regenerative medicine. The results of the study are titled "Wireless Manipulation of Magnetic/Piezoelectric Micromotors for Precise Neural Stem-Like Cell Stimulation", which was published in Advanced Functional Materials (IF =15.621) published online. This work is the result of mutual cooperation with Sun Yat-sen University. Professor Tu Yingfeng from the School of Pharmacy and Professor Peng Fei from the School of Materials Science and Engineering of Sun Yat-sen University are the corresponding authors of this article. Liu Lu, a graduate student of Southern Medical University, is the first author of this article. The research was supported by the National Natural Science Foundation of China and the Guangdong Young Pearl River Scholars Project. Professor Daniela A. Wilson of Nijmegen University participated in this research and is the co-author of the paper.

Central nerve regeneration has always been a major issue in the field of biomedicine. Researchers have discovered that, in addition to non-differentiable mature nerve cells, neural stem cells that maintain the differentiation potential still exist in the central nervous system. If it can promote neural stem cells to differentiate into nerve cells and replace damaged nerve cells, it is expected to repair neural circuits. In recent decades, researchers have used different induction methods such as electrical and chemical signaling molecules (neurotrophic factors) to induce neural stem cell differentiation. However, they face problems such as invasiveness, short half-life, and uncontrollability. Therefore, finding a safer, more effective, and more controllable strategy for inducing neural stem cell differentiation is still a major challenge in neurobiomedicine.
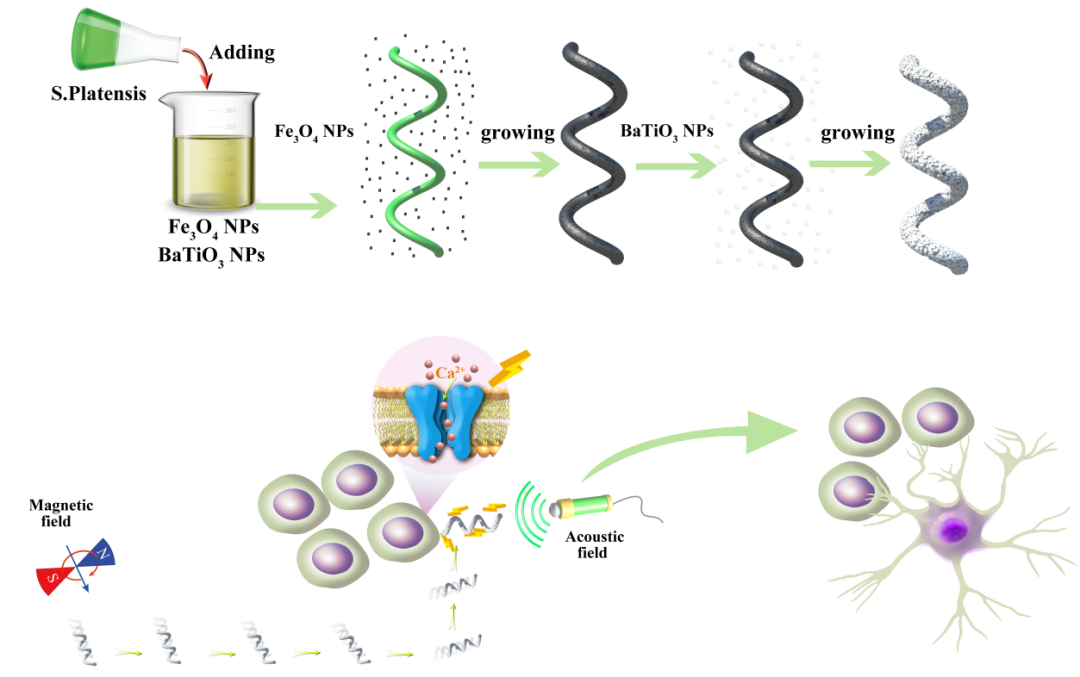
In order to solve this problem, the author of this article took the lead in using biological hybridization technology to construct a multifunctional self-driving micro-nanomotor system with precise induction of neural stem cell differentiation. By introducing magnetically responsive materials into the spiral motor system, the precise movement of the motor under the applied magnetic field is realized, so as to reach the predetermined neural stem cell surface at a fixed point. Combining ultrasound with piezoelectric materials, the mechanical stress generated by ultrasound is used to induce piezoelectric materials on the surface of the motor to generate a direct current output, thereby promoting the formation of axons and differentiation of cells at the stimulation site. This is the first report in the world that uses the magnetron piezoelectric function to precisely induce the precise differentiation of neural stem cells. The system cleverly integrates various functions such as controlled motion and non-invasive induction of neural stem cell differentiation, and realizes precise signal input at the single cell level, which is expected to solve the current challenge of the lack of precise control of neural stem cell differentiation.
Paper link:
https://onlinelibrary.wiley.com/doi/10.1002/advs.201903642
https://onlinelibrary.wiley.com/doi/10.1002/adfm.201910108

In the field of tumor therapy, nanoparticles have attracted widespread attention due to their unique size effect. However, the passive targeting effect of nanoparticles is relatively limited, especially for the delivery of a single chemotherapeutic drug, which tends to cause systemic side effects and has a high tumor recurrence rate. In recent years, people have gradually developed a variety of tumor treatment methods with different characteristics based on chemotherapy, such as photothermal therapy, photodynamic therapy, starvation therapy, etc. However, these single therapies have certain shortcomings that affect the efficacy, and multi-modal collaborative treatment is expected to revolutionize the dilemma faced by tumor treatment.

The team of Professor Yingfeng Tu and the team of Peng Fei of Sun Yat-sen University and the team of Jan van Hest of Eindhoven University of Technology in the Netherlands have designed a multifunctional bionic nanosystem based on gold nanorods. Inspired by the ion chelation between cisplatin and carboxyl groups on hyaluronic acid, the researchers modified the surface of gold nanorods with mercapto-hyaluronic acid, and successfully crosslinked the surface of gold nanorods to form a gel carrier. The drug storehouse enables the classic anticancer drugs doxorubicin and cisplatin to be loaded simultaneously. In addition, in order to achieve a better tumor targeting effect, this research wraps the tumor cell membrane on the surface of the nanocarrier, and finally obtains the biomimetic nanosystem. When co-incubated with homologous and non-homologous tumor cells, the biomimetic nanocarrier can be selectively enriched in homologous tumor cells and exhibits excellent tumor targeting ability. After 24 hours of tail vein injection, the vector achieved a large amount of enrichment in the mouse transplanted tumor. Under the irradiation of the near-infrared laser, the gold nanorods in the carrier core can produce a photothermal effect, and the local thermal energy triggers the release of the loaded chemotherapeutic drugs, thereby achieving coordinated photothermal and chemotherapy for the tumor.
The first author and the last corresponding author of the research paper are both School of Pharmacy, Southern Medical University. The first author of the paper is Gao Junbin, a graduate student of the School of Pharmacy in 2018, and the last corresponding author is Professor Tu Yingfeng; Professor Peng Fei of Sun Yat-sen University Professor Jan van Hest from Eindhoven University of Technology in the Netherlands is the co-corresponding author. The research was supported by the Overseas Youth Project of the Central Organization Department, the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province, and the Pearl River Scholars.
In addition, Professor Tu Yingfeng's team has made new research progress in the field of micro-nanomotor regenerative medicine. The results of the study are titled "Wireless Manipulation of Magnetic/Piezoelectric Micromotors for Precise Neural Stem-Like Cell Stimulation", which was published in Advanced Functional Materials (IF =15.621) published online. This work is the result of mutual cooperation with Sun Yat-sen University. Professor Tu Yingfeng from the School of Pharmacy and Professor Peng Fei from the School of Materials Science and Engineering of Sun Yat-sen University are the corresponding authors of this article. Liu Lu, a graduate student of Southern Medical University, is the first author of this article. The research was supported by the National Natural Science Foundation of China and the Guangdong Young Pearl River Scholars Project. Professor Daniela A. Wilson of Nijmegen University participated in this research and is the co-author of the paper.

Central nerve regeneration has always been a major issue in the field of biomedicine. Researchers have discovered that, in addition to non-differentiable mature nerve cells, neural stem cells that maintain the differentiation potential still exist in the central nervous system. If it can promote neural stem cells to differentiate into nerve cells and replace damaged nerve cells, it is expected to repair neural circuits. In recent decades, researchers have used different induction methods such as electrical and chemical signaling molecules (neurotrophic factors) to induce neural stem cell differentiation. However, they face problems such as invasiveness, short half-life, and uncontrollability. Therefore, finding a safer, more effective, and more controllable strategy for inducing neural stem cell differentiation is still a major challenge in neurobiomedicine.

In order to solve this problem, the author of this article took the lead in using biological hybridization technology to construct a multifunctional self-driving micro-nanomotor system with precise induction of neural stem cell differentiation. By introducing magnetically responsive materials into the spiral motor system, the precise movement of the motor under the applied magnetic field is realized, so as to reach the predetermined neural stem cell surface at a fixed point. Combining ultrasound with piezoelectric materials, the mechanical stress generated by ultrasound is used to induce piezoelectric materials on the surface of the motor to generate a direct current output, thereby promoting the formation of axons and differentiation of cells at the stimulation site. This is the first report in the world that uses the magnetron piezoelectric function to precisely induce the precise differentiation of neural stem cells. The system cleverly integrates various functions such as controlled motion and non-invasive induction of neural stem cell differentiation, and realizes precise signal input at the single cell level, which is expected to solve the current challenge of the lack of precise control of neural stem cell differentiation.
Paper link:
https://onlinelibrary.wiley.com/doi/10.1002/advs.201903642
https://onlinelibrary.wiley.com/doi/10.1002/adfm.201910108
18915694570
Previous: Use perovskite diodes


