Cell Stem Cell: Hydrogel network dynamics regulate vascular morphogenesis
Matrix dynamics will affect the development of single cells into complex multicellular tissues. However, little is known about the mechanism by which matrix dynamics regulate tissue assembly. Hydrogels are often used to simulate 3D physiological tissue matrix due to their characteristics very similar to extracellular matrix. So, how does dynamic hydrogel affect tissue formation?
Recently, Professor Sharon Gerechts team from Hopkins University in the United States published a research paper entitled "Hydrogel Network Dynamics Regulate Vascular Morphogenesis" on Cell Stem Cell. The researchers constructed a viscoelastic hydrogel system through dynamic covalent cross-linking, and determined the role of the dynamic network matrix and its potential mechanism for regulating vascular tissue morphogenesis (Figure 1): The dynamic hydrogel network enhances cell contractility It aggregates integrins, recruits focal adhesion proteins and promotes the formation of large focal adhesions, thereby rapidly forming vascular networks; while static hydrogel prevents integrin clustering and the formation and development of vascular beds.
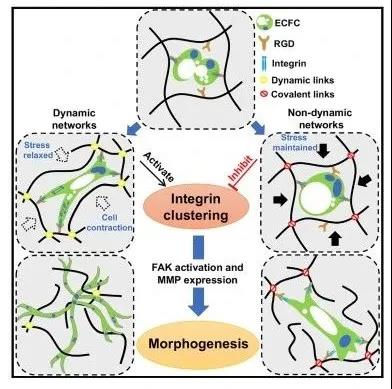
Figure 1 Matrix dynamics regulate the formation mechanism of vascular tissue First, the researchers introduced the design and characterization of the viscoelastic hydrogel system. Use a hydrogel composed of gelatin and dextran, in which gelatin is the main component; dynamic covalent imine and acylhydrazone form a dynamic network hydrogel (D-hydrogel), the control group is static covalent methacrylate (MAs) static hydrogels (N-hydrogels) formed. The researchers not only controlled the stiffness of the hydrogel by controlling the cross-linking conditions, but also evaluated the modulus and stress relaxation curve of different hydrogels before and after the culture medium (Figure 2).
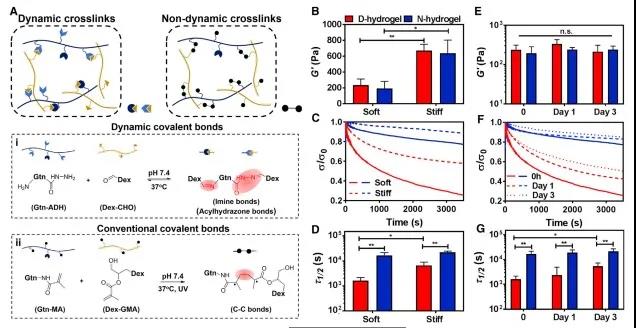
Figure 2 Design and characterization of dynamic network hydrogels (D-hydrogels) and non-dynamic network hydrogels (N-hydrogels) Next, the role of dynamic networks and the regulation of vascular tissue morphology are demonstrated from the following five aspects The mechanism of occurrence.
Dynamic network hydrogel promotes the rapid formation of blood vessel morphology. Use human endothelial colony forming cells and soft hydrogel (~200pa). After eliminating the interference of network nutrient transmission, cytotoxicity and other influencing factors, it is proved that the dynamic hydrogel network can promote the formation of blood vessel tissue faster (Figure 3).
Dynamic network hydrogels promote the formation of focal adhesion protein (FA) and the aggregation of integrins. The effect of hydrogel network on cell germination and branching is the key to the formation of viscous tissue structure. The researchers proved that the D-hydrogel network is easier to deform and has stronger traction, and the encapsulated cells have greater traction on the matrix, which promotes the interaction between integrins and the hydrogel network, thereby promoting the aggregation of integrins. The accumulation of integrin β1 promotes the recruitment of focal adhesion proteins to form stable focal adhesions.
Hydrogel network relaxation is a necessary condition for contraction-mediated integrin aggregation and subsequent blood vessel formation. After the initial formation of small FAs, the cell contractility of pMLC-mediated actin contraction promotes integrin aggregation, FAs recruitment, and then activates downstream signaling events, thereby promoting downstream signaling and vascular morphogenesis. However, the non-dynamic hydrogel prevents myosin-mediated integrin aggregation, thus, leading to ineffective sprouting and blood vessel formation.
The dynamic network leads to the activation of FA kinase (FAK), the up-regulation of MMP expression and the deposition of new ECM. D-hydrogel network supports the formation and activation of FAs and FAK phosphorylation in encapsulated ECFCs; D-hydrogel environment can promote the expression of MMPs in ECFCs, thereby promoting matrix remodeling and degradation, allowing cells to invade the surrounding environment and Build blood vessel tissue.
FAK activation regulates the accumulation of integrins, cell contractility and subsequent formation of the vascular bed. FAK plays an important role in maintaining and regulating FA stability. In addition, FAK activation promotes MLC phosphorylation and regulates integrin expression, promotes integrin aggregation, and FAK participates in integrin-mediated downstream signal transduction, leading to the up-regulation of various MMPs, thereby causing vascular morphogenetic cells to germinate, branch and cell migration .
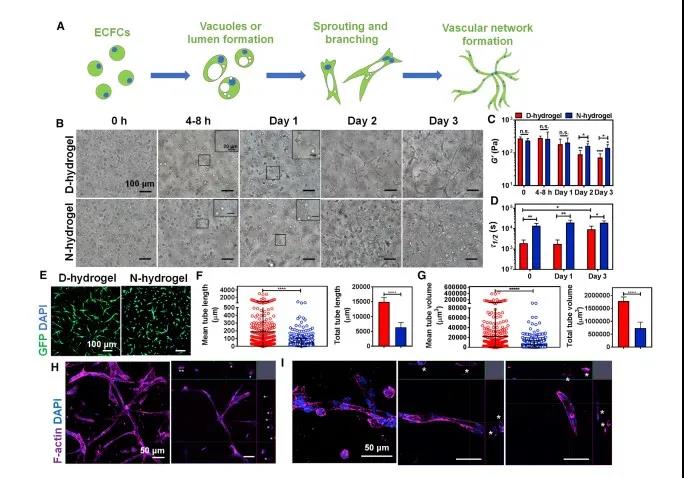
Figure 3 Hydrogels with dynamic and non-dynamic networks have different regulatory effects on the angiogenesis of endothelial colony forming cells
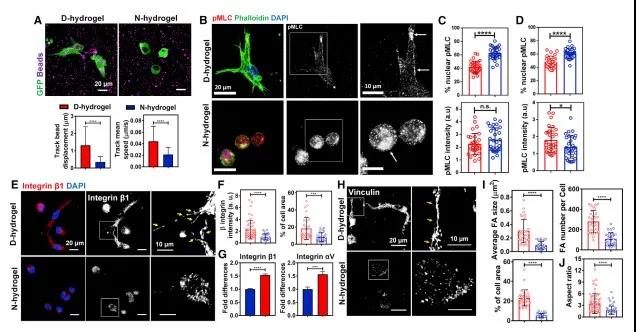
Figure 4 Dynamic network hydrogel promotes FA formation
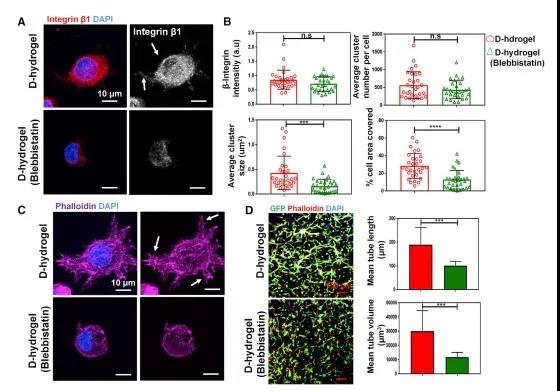
Figure 5 Cell contraction mediates integrin aggregation and angiogenesis
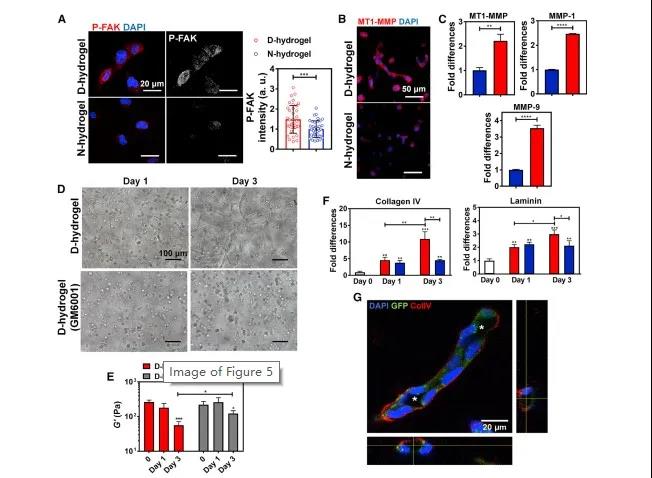
Figure 6 Dynamic network leads to FAK activation and matrix degradation by increasing MMP expression and ECM deposition
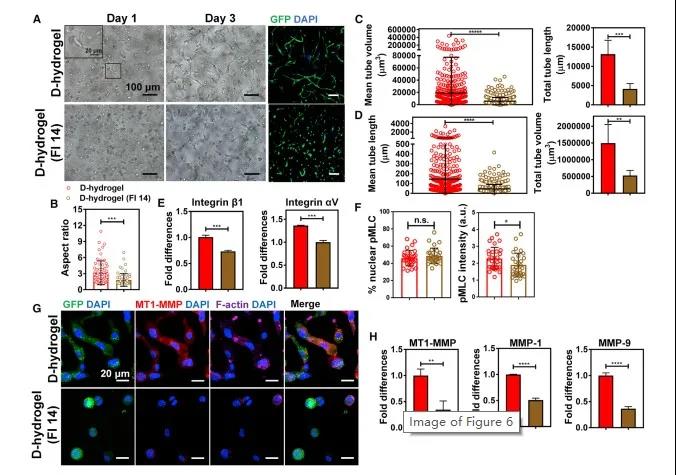
Figure 7 FAK activation in ECFCs is a necessary condition for D-hydrogel remodeling and vascular morphogenesis. Finally, the in vivo angiogenesis mechanism and angiogenesis effect of the dynamic network were studied. The results of in vivo experiments are consistent with the results of in vitro experiments: dynamic hydrogels have a higher cell density than static hydrogels, with more sprouting and branching cells; dynamic hydrogel networks degrade faster and produce more blood vessels. In addition, the dynamic hydrogel not only promotes the rapid formation of blood vessels in the body, but also promotes the regeneration and circulation of host blood vessels (Figure 8).
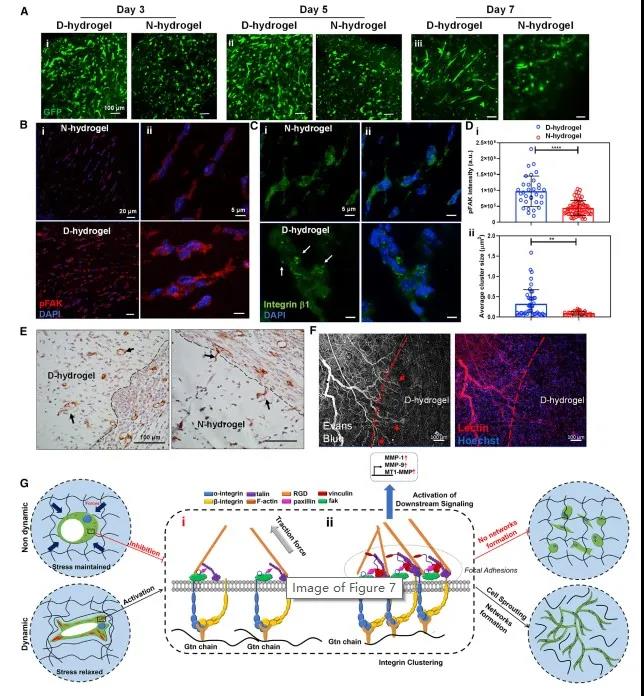
Figure 8 Dynamic network accelerates angiogenesis in vivo and proposes a molecular pathway for ECFCs to respond to the dynamic network. In summary, matrix dynamics is the key to vascular tissue assembly. This research not only helps us understand how hydrogel network dynamics affects vascular morphogenesis, it can also be used to study complex tissue assembly, morphogenesis, and the design of biological hydrogel material systems f
Recently, Professor Sharon Gerechts team from Hopkins University in the United States published a research paper entitled "Hydrogel Network Dynamics Regulate Vascular Morphogenesis" on Cell Stem Cell. The researchers constructed a viscoelastic hydrogel system through dynamic covalent cross-linking, and determined the role of the dynamic network matrix and its potential mechanism for regulating vascular tissue morphogenesis (Figure 1): The dynamic hydrogel network enhances cell contractility It aggregates integrins, recruits focal adhesion proteins and promotes the formation of large focal adhesions, thereby rapidly forming vascular networks; while static hydrogel prevents integrin clustering and the formation and development of vascular beds.

Figure 1 Matrix dynamics regulate the formation mechanism of vascular tissue First, the researchers introduced the design and characterization of the viscoelastic hydrogel system. Use a hydrogel composed of gelatin and dextran, in which gelatin is the main component; dynamic covalent imine and acylhydrazone form a dynamic network hydrogel (D-hydrogel), the control group is static covalent methacrylate (MAs) static hydrogels (N-hydrogels) formed. The researchers not only controlled the stiffness of the hydrogel by controlling the cross-linking conditions, but also evaluated the modulus and stress relaxation curve of different hydrogels before and after the culture medium (Figure 2).

Figure 2 Design and characterization of dynamic network hydrogels (D-hydrogels) and non-dynamic network hydrogels (N-hydrogels) Next, the role of dynamic networks and the regulation of vascular tissue morphology are demonstrated from the following five aspects The mechanism of occurrence.
Dynamic network hydrogel promotes the rapid formation of blood vessel morphology. Use human endothelial colony forming cells and soft hydrogel (~200pa). After eliminating the interference of network nutrient transmission, cytotoxicity and other influencing factors, it is proved that the dynamic hydrogel network can promote the formation of blood vessel tissue faster (Figure 3).
Dynamic network hydrogels promote the formation of focal adhesion protein (FA) and the aggregation of integrins. The effect of hydrogel network on cell germination and branching is the key to the formation of viscous tissue structure. The researchers proved that the D-hydrogel network is easier to deform and has stronger traction, and the encapsulated cells have greater traction on the matrix, which promotes the interaction between integrins and the hydrogel network, thereby promoting the aggregation of integrins. The accumulation of integrin β1 promotes the recruitment of focal adhesion proteins to form stable focal adhesions.
Hydrogel network relaxation is a necessary condition for contraction-mediated integrin aggregation and subsequent blood vessel formation. After the initial formation of small FAs, the cell contractility of pMLC-mediated actin contraction promotes integrin aggregation, FAs recruitment, and then activates downstream signaling events, thereby promoting downstream signaling and vascular morphogenesis. However, the non-dynamic hydrogel prevents myosin-mediated integrin aggregation, thus, leading to ineffective sprouting and blood vessel formation.
The dynamic network leads to the activation of FA kinase (FAK), the up-regulation of MMP expression and the deposition of new ECM. D-hydrogel network supports the formation and activation of FAs and FAK phosphorylation in encapsulated ECFCs; D-hydrogel environment can promote the expression of MMPs in ECFCs, thereby promoting matrix remodeling and degradation, allowing cells to invade the surrounding environment and Build blood vessel tissue.
FAK activation regulates the accumulation of integrins, cell contractility and subsequent formation of the vascular bed. FAK plays an important role in maintaining and regulating FA stability. In addition, FAK activation promotes MLC phosphorylation and regulates integrin expression, promotes integrin aggregation, and FAK participates in integrin-mediated downstream signal transduction, leading to the up-regulation of various MMPs, thereby causing vascular morphogenetic cells to germinate, branch and cell migration .

Figure 3 Hydrogels with dynamic and non-dynamic networks have different regulatory effects on the angiogenesis of endothelial colony forming cells

Figure 4 Dynamic network hydrogel promotes FA formation

Figure 5 Cell contraction mediates integrin aggregation and angiogenesis

Figure 6 Dynamic network leads to FAK activation and matrix degradation by increasing MMP expression and ECM deposition

Figure 7 FAK activation in ECFCs is a necessary condition for D-hydrogel remodeling and vascular morphogenesis. Finally, the in vivo angiogenesis mechanism and angiogenesis effect of the dynamic network were studied. The results of in vivo experiments are consistent with the results of in vitro experiments: dynamic hydrogels have a higher cell density than static hydrogels, with more sprouting and branching cells; dynamic hydrogel networks degrade faster and produce more blood vessels. In addition, the dynamic hydrogel not only promotes the rapid formation of blood vessels in the body, but also promotes the regeneration and circulation of host blood vessels (Figure 8).

Figure 8 Dynamic network accelerates angiogenesis in vivo and proposes a molecular pathway for ECFCs to respond to the dynamic network. In summary, matrix dynamics is the key to vascular tissue assembly. This research not only helps us understand how hydrogel network dynamics affects vascular morphogenesis, it can also be used to study complex tissue assembly, morphogenesis, and the design of biological hydrogel material systems f
18915694570
Previous: Tianjin University


