In-situ polymerization strategy makes MOF an anti-cancer
Article introduction:
MOFs have the advantages of structural controllability, porosity adjustable, and easy functional modification, making them a good carrier for nanomedicine, but their physiological stability is poor, which severely limits their application in biomedicine. Zinc-based MOFs have poor stability in water, especially in acidic water buffers. Pickaxe-based MOFs are very sensitive to phosphate-containing buffers, such as phosphate buffered saline (PBS) and RPMI-1640 medium, both of which have a higher phosphate ion concentration. This sensitivity is due to Zr ions and guest There is a strong binding affinity between phosphate ions. The metal-free COFs have strong stability but poor biocompatibility and are not suitable for biochemical research.
In order to promote the biological application of MOFs, the functionalization of the outer surface of MOFs nanoparticles has been achieved through coordinated binding on unsaturated metal sites, covalent binding with pre-functionalized linkers, and ligand exchange. Although the functionalization of the outer surface has been successful, so far there are only a few easy-to-operate and generalizable intracellular drug release systems that are responsive to stimulation. Although phosphate ions can trigger the decomposition of many Zr-based MOFs, phosphate-responsive drug delivery systems using uniformly porous MOFs nanoparticles as a carrier have not been reported because physiological phosphate ions cannot control the decomposition of MOFs. Especially in the internal environment. Therefore, Zhantong Wang et al. designed a ZrMOF with high physiological stability and strong phosphate stimulation response function to make MOFs further in biomedical applications.
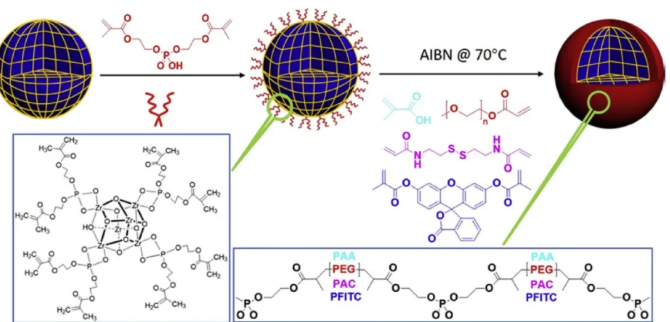
The novel MOFs nanoparticle in-situ polymerization strategy, that is, to wrap the nanoparticles with functionalized polymers, not only provides intrinsic stability for the wrapped nanoparticles, but also provides a stimulus-responsive decomposition mechanism for the nanoparticles. In order to wrap the functional polymers of different monomers on the surface of MOF nanoparticles, the author first fixed BMAP on the surface of MOFs, and then introduced free radical initiator AIBN to initiate polymerization on BMAP to form a surface coating, thereby preparing different polymers Protected MOFs.
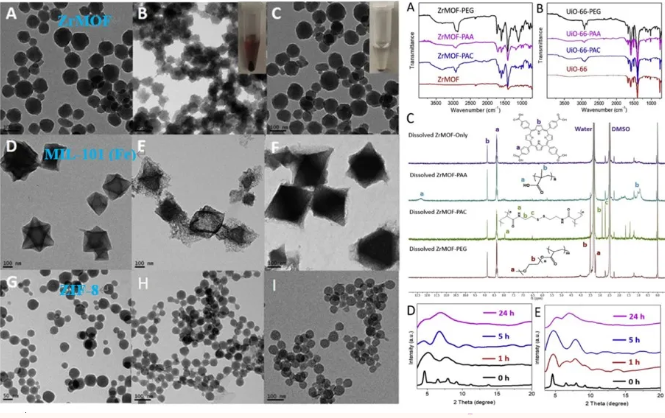
The author used FTIR, NMR and XRD patterns to characterize the MOFs nanoparticles successfully encapsulated by different polymers, and used TEM images to explore the stability of different MOF nanoparticles in different buffers. It can be seen that the stability of the encapsulated polymer is significantly improved. .
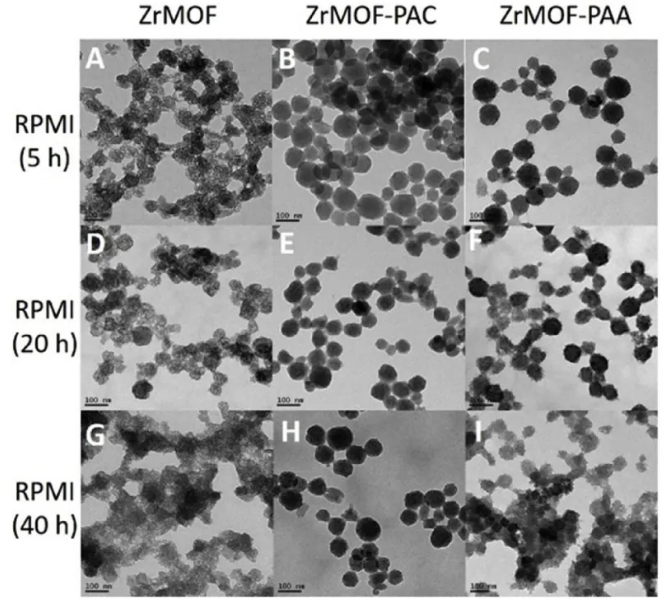
The stability of ZrMOF in cell culture medium is very important and must be considered when conducting cell viability studies. Therefore, the stability of ZrMOF-only, ZrMOF-PAA and ZrMOF-PAC in the commonly used cell culture medium RPMI-1640 was further tested. And ZrMOF-PAC is the most stable of the three encapsulated ZrMOF.
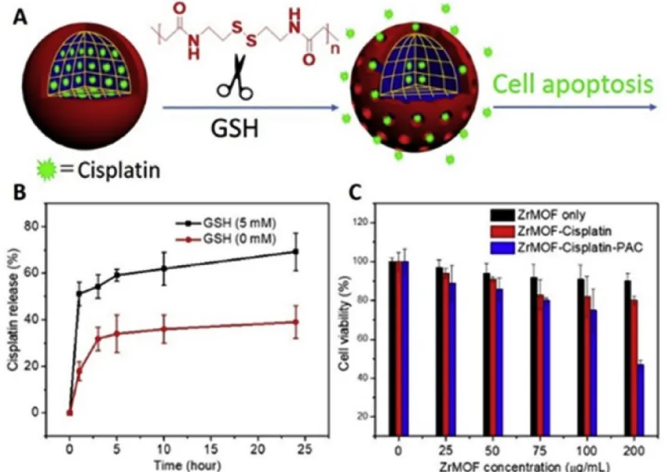
The surface polymers of MOFs nanoparticles not only increase their stability in buffers and media, but also provide functions such as stimulating drug delivery. The author used ZrMOF-PAC loaded with the chemotherapeutic drug cisplatin to conduct intracellular drug delivery experiments and first demonstrated the GSH-stimulus responsiveness of ZrMOF-PAC, because GSH is the trigger to initiate PAC decomposition. In the case of GSH, according to ICP analysis, it can be seen that 50% of cisplatin is released from ZrMOF-PAC, and 20% of cisplatin is gradually released due to phosphate ion etching and the decomposition of ZrMOF; while without GSH, Cisplatin is gradually released over time. Compared with ZrMOF-PAC triggered by GSH, nearly 30% of cisplatin is trapped in ZrMOF-PAC nanoparticles. Secondly, using MTT to detect the anti-cancer activity of ZrMOF, the study found that when there is no PAC coating, the cisplatin loaded after the buffer leaks seriously, and the cancer treatment effect is relatively poor; while the polymer-coated ZrMOF-PAC can prevent the cisplatin from being removed from the nano Leak in ZrMOF to ensure effective delivery of drugs. After cell uptake, the high level of GSH in cancer cells decomposes the cross-linked PAC polymer on the surface of ZrMOF, allowing the smooth release of cisplatin from MOF. At the same time, the phosphate ion in the cell etches ZrMOF and accelerates the release of the drug, thereby showing a better cancer treatment effect.
Conclusion: The author designed a new type of MOFs in-situ polymerization strategy to significantly enhance the stability of different nano-sized MOFs (ZrMOF, MIL-101, ZIF-8 and Uio-66) under physiological conditions in vivo and in vitro, especially In phosphate solution. In-situ polymerization enables the prodrugs to not only increase the stability of nano-MOFs, but also release drugs in response to the cells, providing a stable, efficient and safe drug delivery platform. The influence of buffer media such as phosphate on MOFs provides new ideas for solving problems in our material synthesis and application.
Note: All the graphs in the tweet are extracted from the original text. This article is only for the study of literature reviews (the level is limited, if there are errors, please forgive me).
Information source: Gao Le ZhanGroup
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
MOFs have the advantages of structural controllability, porosity adjustable, and easy functional modification, making them a good carrier for nanomedicine, but their physiological stability is poor, which severely limits their application in biomedicine. Zinc-based MOFs have poor stability in water, especially in acidic water buffers. Pickaxe-based MOFs are very sensitive to phosphate-containing buffers, such as phosphate buffered saline (PBS) and RPMI-1640 medium, both of which have a higher phosphate ion concentration. This sensitivity is due to Zr ions and guest There is a strong binding affinity between phosphate ions. The metal-free COFs have strong stability but poor biocompatibility and are not suitable for biochemical research.
In order to promote the biological application of MOFs, the functionalization of the outer surface of MOFs nanoparticles has been achieved through coordinated binding on unsaturated metal sites, covalent binding with pre-functionalized linkers, and ligand exchange. Although the functionalization of the outer surface has been successful, so far there are only a few easy-to-operate and generalizable intracellular drug release systems that are responsive to stimulation. Although phosphate ions can trigger the decomposition of many Zr-based MOFs, phosphate-responsive drug delivery systems using uniformly porous MOFs nanoparticles as a carrier have not been reported because physiological phosphate ions cannot control the decomposition of MOFs. Especially in the internal environment. Therefore, Zhantong Wang et al. designed a ZrMOF with high physiological stability and strong phosphate stimulation response function to make MOFs further in biomedical applications.
The novel MOFs nanoparticle in-situ polymerization strategy, that is, to wrap the nanoparticles with functionalized polymers, not only provides intrinsic stability for the wrapped nanoparticles, but also provides a stimulus-responsive decomposition mechanism for the nanoparticles. In order to wrap the functional polymers of different monomers on the surface of MOF nanoparticles, the author first fixed BMAP on the surface of MOFs, and then introduced free radical initiator AIBN to initiate polymerization on BMAP to form a surface coating, thereby preparing different polymers Protected MOFs.

The author used FTIR, NMR and XRD patterns to characterize the MOFs nanoparticles successfully encapsulated by different polymers, and used TEM images to explore the stability of different MOF nanoparticles in different buffers. It can be seen that the stability of the encapsulated polymer is significantly improved. .

The stability of ZrMOF in cell culture medium is very important and must be considered when conducting cell viability studies. Therefore, the stability of ZrMOF-only, ZrMOF-PAA and ZrMOF-PAC in the commonly used cell culture medium RPMI-1640 was further tested. And ZrMOF-PAC is the most stable of the three encapsulated ZrMOF.

The surface polymers of MOFs nanoparticles not only increase their stability in buffers and media, but also provide functions such as stimulating drug delivery. The author used ZrMOF-PAC loaded with the chemotherapeutic drug cisplatin to conduct intracellular drug delivery experiments and first demonstrated the GSH-stimulus responsiveness of ZrMOF-PAC, because GSH is the trigger to initiate PAC decomposition. In the case of GSH, according to ICP analysis, it can be seen that 50% of cisplatin is released from ZrMOF-PAC, and 20% of cisplatin is gradually released due to phosphate ion etching and the decomposition of ZrMOF; while without GSH, Cisplatin is gradually released over time. Compared with ZrMOF-PAC triggered by GSH, nearly 30% of cisplatin is trapped in ZrMOF-PAC nanoparticles. Secondly, using MTT to detect the anti-cancer activity of ZrMOF, the study found that when there is no PAC coating, the cisplatin loaded after the buffer leaks seriously, and the cancer treatment effect is relatively poor; while the polymer-coated ZrMOF-PAC can prevent the cisplatin from being removed from the nano Leak in ZrMOF to ensure effective delivery of drugs. After cell uptake, the high level of GSH in cancer cells decomposes the cross-linked PAC polymer on the surface of ZrMOF, allowing the smooth release of cisplatin from MOF. At the same time, the phosphate ion in the cell etches ZrMOF and accelerates the release of the drug, thereby showing a better cancer treatment effect.

Conclusion: The author designed a new type of MOFs in-situ polymerization strategy to significantly enhance the stability of different nano-sized MOFs (ZrMOF, MIL-101, ZIF-8 and Uio-66) under physiological conditions in vivo and in vitro, especially In phosphate solution. In-situ polymerization enables the prodrugs to not only increase the stability of nano-MOFs, but also release drugs in response to the cells, providing a stable, efficient and safe drug delivery platform. The influence of buffer media such as phosphate on MOFs provides new ideas for solving problems in our material synthesis and application.
Note: All the graphs in the tweet are extracted from the original text. This article is only for the study of literature reviews (the level is limited, if there are errors, please forgive me).
Information source: Gao Le ZhanGroup
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
18915694570
Previous: Professor Xiaowen Shi


