Review of Mater. Horiz. from Ghent University, Belgium: Biologically inspired double network hydrogel
The functions of living organisms are constructed in a bottom-up approach from the atomic level to the macro level, and can continuously adapt to harsh environments. Some marine organisms can absorb large amounts of water while maintaining their structural integrity and mechanical properties. The soft tissue of the human body can effectively disperse and disperse mechanical stress, and at the same time withstand greater deformation. Their excellent endurance is related to their anisotropic mechanical behavior and is caused by subtle reversible and/or non-covalent interactions. This reversible toughening mechanism is based on sacrificial bonds and the unfolding of ordered domains in the organic components of the gel-like organization. Hydrogel has become the most representative biomaterial for tissue engineering due to its biocompatibility and similarity with biological soft tissue. The dual network (DN) hydrogel composed of two mutually cross-linked networks was proposed by Gong Jianping of Hokkaido University, Japan. It has both high strength and toughness. Compared with conventional DN hydrogels, bio-inspired DN hydrogels can imitate biological structures of different length scales, can quickly self-heal when damaged, have good biocompatibility, cell interaction and injectability. Although significant progress has been made in the study of bio-inspired DN hydrogels, reports of bio-inspired DN hydrogels that solve practical problems are still limited. Based on this, Richard Hoogenboom of Ghent University reviewed the design, synthesis and application of bio-inspired DN hydrogels in various fields (Figure 1), as well as future challenges and prospects. The article Bioinspired double network hydrogels: from covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels was published in "Mater. Horiz." (DOI: 10.1039/D0MH01514H)

Figure 1. Application of bio-inspired DN hydrogel. [Picture introduction] 1. Bio-inspired DN hydrogel due to the dynamic nature of the sacrificial bond, DN hydrogel has toughness and self-repairing ability, but this sacrificial bond is mainly based on irreversibly broken covalent bonds. A variety of DN hydrogels have been reported so far (Figure 2).
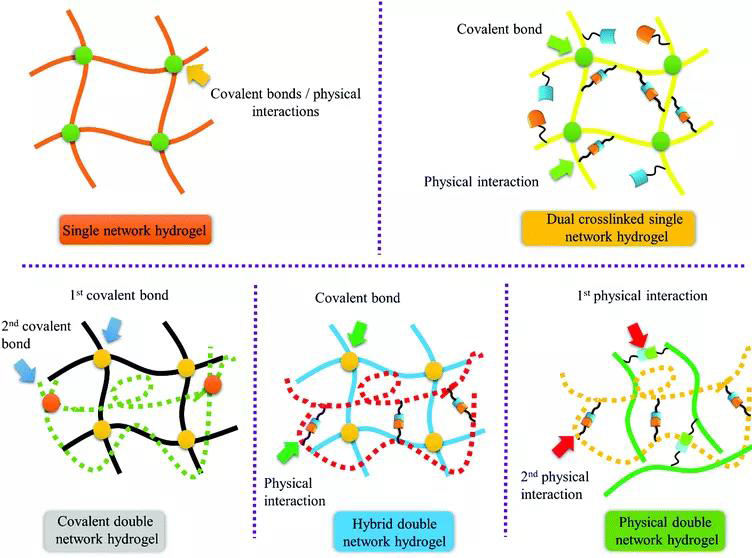
Figure 2. Different types of bio-inspired hydrogels. 2. Covalent double network hydrogel DN hydrogel is synthesized by a two-step sequential polymerization method. The rigid and brittle network formed in the first step is covalently cross-linked. The loosely cross-linked, soft and flexible network formed in the second step is formed inside the first network. The DN hydrogel made by this method has extremely high strength and toughness. However, the first network greatly limits the application of DN hydrogel in biological systems. To overcome this problem, Gong Jianping developed a general method for synthesizing DN hydrogels, in which the sacrificial network is composed of a neutral polymer using a "molecular scaffold", and linear polyelectrolyte chains or ionic micelles are introduced into the first network To increase the osmotic pressure of the composite neutral gel, resulting in a highly swollen, weak and brittle gel. Then the DN hydrogel is obtained by polymerizing the second precursor solution (Figure 3).
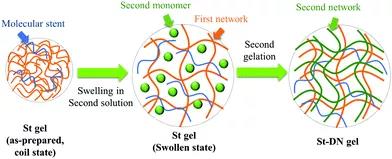
Figure 3 How the polyelectrolyte polymer promotes the formation of a double-network hydrogel from neutral polymers through the "molecular scaffold" strategy. Gong Jianpings team also recently developed a covalently cross-linked DN hydrogel that can resist multiple mechanical stress cycles (Figure 4). The covalent cross-links in the gel can be mechanically destroyed, and at the same time free radicals are generated through mechanochemistry, and a new network is constructed by externally supplying monomers and cross-linking agents. By using functional monomers in the deformation strengthening process, DN hydrogels can be given responsiveness. In addition, covalent DN hydrogels used as artificial organisms (such as stents that induce autonomous cartilage regeneration in vivo) have been reported; covalent DN hydrogels whose mechanical properties will not change after liquid washing and heat sterilization; swelling properties, Covalent DN hydrogel with extremely high strength and physical cross-linking bonds between electrostatic networks.
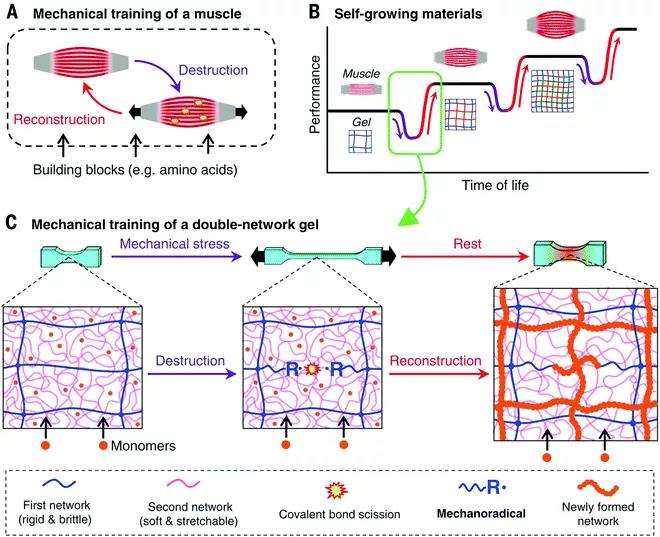
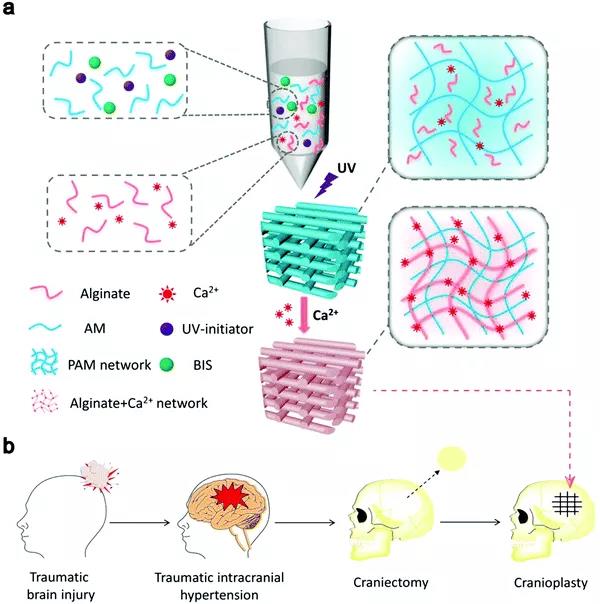
Figure 5 (a) Synthesis strategy of alginate-Ca 2+ /PAAm hybrid DN hydrogel. (B) Severe head injury treated with hybrid DN hydrogel.
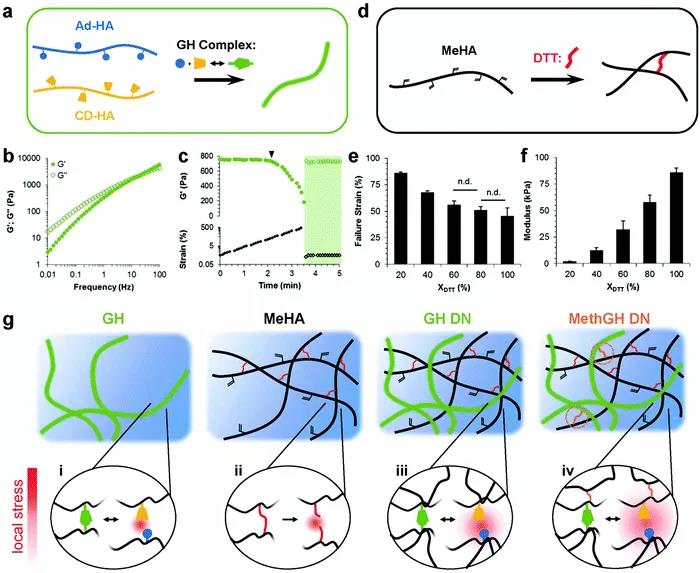
Figure 6 Schematic diagram of different types of hydrogels. Physical DN hydrogel. Although hybrid DN hydrogels have good mechanical strength and toughness, they cannot self-repair due to the slow diffusion of polymer chains. In addition, due to the inevitable rupture of covalent bonds in the chemical cross-linking network, irreversible damage cannot be completely suppressed. The two networks of physical DN hydrogels are formed based on two orthogonal types of non-covalent interactions to produce tough and fast self-healing hydrogels. In contrast to covalent DN hydrogels and hybrid DN hydrogels, physical DN hydrogels are based on the "reversible chain association-dissociation" mechanism in two networks to achieve toughness and self-healing properties. In this way, both physically cross-linked networks can participate in energy dissipation during hydrogel deformation. The first type: physical DN hydrogel based on coordination of metal ions. Inspired by natural systems, the reversibility and coordination of non-covalent metal ions as a sacrificial bond is a method of making physical DN hydrogels with high mechanical strength and toughness, which has attracted more and more attention. Mechanical properties such as processability, dynamic adaptability and self-healing ability (Figure 7).
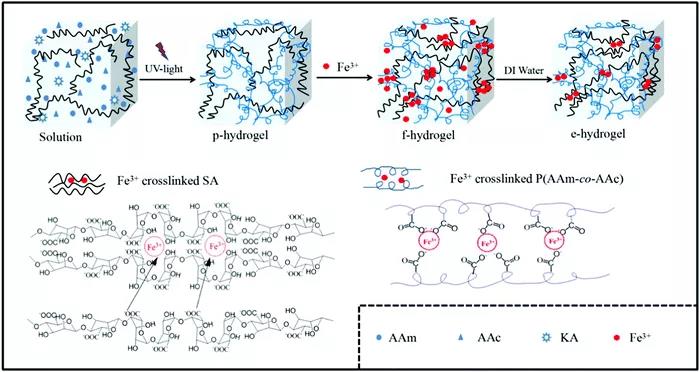
Figure 7 Sodium alginate/poly(acrylamide-co-acrylic acid)/Fe3+(SA/P(AAm-co-AAc)) DN hydrogel. The second type: physical DN hydrogel based on hydrogen bonds. Usually the second network component associates with the hydrophilic mid-block of the first network through hydrogen bonding. Even if no cross-linked network is formed, this association results in its immobilization in the physical DN hydrogel. When deformed, the hydrogen bonds break and dissipate energy, resulting in a tough physical DN hydrogel. Compared with conventional DN hydrogels, in terms of network stability and mechanical strength, hydrogen-bonded physical DN hydrogels are promising materials. The third type: physical DN hydrogel based on hydrophobic interaction. Hydrophobic association can act as a reversible chain association-dissociation network when preparing tough and self-healing DN hydrogels. Because the dynamic physical cross-linking of hydrophobic segments and latex particles will dissipate a lot of energy, the physical DN hydrogel has excellent mechanical properties.
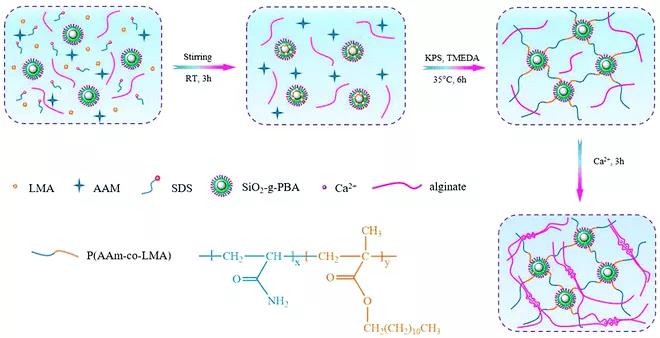
Figure 8. Synthesis of hydrophobically associated crosslinked PAAm (HPAAm)–HLP/alginate–Ca2+ DN hydrogel with a dual physical crosslink network. The fourth type: physical DN hydrogel based on host-guest interaction. Although the interaction between host and guest has received wide attention as the driving force of supramolecular chemistry and advanced materials, most of the research on supramolecular hydrogels is limited to the preparation of physical single network hydrogels and double Cross-linked single network hydrogel. Therefore, the development of host-guest complex as a reversible association-dissociation bond, rapid self-repair, injectability and cytocompatibility of physical DN hydrogel is still challenging. Challenges of bio-inspired DN hydrogels The author finally discussed the challenges and successes faced in the development of bio-inspired DN hydrogels and their potential progress in biomedical applications. 1. Bioinspired DN hydrogels with biocompatibility or shear thinning and self-healing properties are still in their infancy. 2. The reproducibility of bioinspired DN hydrogels based on natural polymers is a key issue that should be solved in the future, because these polymers have different characteristics and there are differences between batches. 3. The construction of biocompatible and functional DN hydrogels with similar properties as biological systems still faces challenges. 4. The development of tough physical DN hydrogels remains a major challenge in this region. 5. Development of DN hydrogel with good adhesion and injectability is the main challenge for its biomedical applications. 6. Combine the multifunctional bio-inspired DN hydrogel with another function (such as electrical conductivity, magnetic or luminescence properties), so that it can be used in flexible strain sensors, thermomechanical sensors, dual sensors and electroluminescence Equipment is a very hot research field in the future.
Source of information: Frontiers of Polymer Science
This information is from the Internet for academic exchanges. If there is any infringement, please contact us and delete it immediately
18915694570
Previous: Review of Mater. Horiz


